Coronavirus (COVID-19) Symptoms, Treatment, and Recent Medical Challenges to the World: A Review
Muhammad Asif*, Amir Sohail, Akasha Muqadas, Muhammad Shahzeb Khan, Alina Niazi
Amir Sohail1, Akasha Muqadas2, Muhammad Shahzeb Khan3, Alina Niazi4 and Muhammad Asif5*
1Department of Chemistry, University of Sargodha, Pakistan
2Department of Zoology, University of Mianwali, Punjab, Pakistan
3Sulaiman Bin Abdullah Aba Al-Khail Centre for Interdisciplinary Research in Basic Sciences (SA-CIRBS), International Islamic University Islamabad, Pakistan
4Department of Chemistry, University of Mianwali, Punjab, Pakistan
5Institute of Energy and Environmental Engineering, University of the Punjab, Lahore-54000, Pakistan
- Corresponding Author:
- Muhammad Asif
Institute of Energy and Environmental Engineering,
University of the Punjab, Lahore-54000, Pakistan
Tel: +923047092647
E-mail: amal.elfakhri@uob.edu.ly
Received date: August 18, 2020; Accepted date: October 29, 2020; Published date: November 05, 2020
Citation: Sohail A, Muqadas A, Khan MS, Niazi AS, Asif M (2020) Coronavirus (COVID-19) Symptoms, Treatment and Recent Medical Challenges to the World: A Review. J Prev Med Vol. 5 Iss No. 4: 20.
Abstract
Coronavirus infections have emerged as epidemic and pandemic threats throughout the world. Severe acute respiratory syndrome coronavirus (SARS-CoV-2) has spread across 203 countries and territories in all 5 major continents. World Health Organization (WHO) declared this as a public health emergency of international concern on January 30, 2020. Subsequently, on February 11, 2020, a new name was given to this disease i.e. COVID-19 by an expert group from WHO. As of 3:37 pm CEST, 14 August 2020, there have been 20,730,456 confirmed cases of COVID-19, including 751,154 deaths, reported to WHO. It possibly originated from a small animal market in Wuhan, China. A cluster of patients was admitted with unusual pneumonia not responding to treatment in various hospitals. Epidemiological, genomic analysis and correlation with other coronaviruses led to the isolation of new coronavirus, closely resembling the bat coronaviruses, from such patients in Wuhan. They were identified as the SARS-CoV-2. This virus infection presents an influenza-like illness in the affected people. Fever, cough, respiratory distress with fatigue, diarrhea, nausea, and vomiting are common symptoms seen in adults. The transmissibility of SARS-CoV-1 was less as compared to SARS-CoV-2 infection, and it was well controlled with good public health efforts. The present COVID-19 epidemic is still in the acceleration phase of 3 and 4 in various countries. Without any effective antiviral agents available at present, the need of the hour is early case detection, isolation of cases, use of good preventive care measures by the household contacts and in the hospital setup. Here, we have placed the symptoms and treatment schemes for the COVID-19 from the practice of different research organizations of the World including WHO.
Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is the causative agent of coronavirus disease 2019 (COVID19) [1]. On March 11, 2020, the World Health Organization (WHO) elevated the status of novel coronavirus (COVID19) to a pandemic [2]. SARS CoV-2 was discovered in December 2019 in Wuhan City, Hubei Province, China [3]. SARS-CoV-2 is declared to have phylogenetic similarity to severe acute respiratory syndrome coronavirus [1]. Many patients were detected with the symptoms like pneumonia from the city of Wuhan in Hubei province in China. Coronaviruses (COVs) are the largest group of viruses belonging to the Coronaviridae family. COVID19 affects both upper and lower respiratory tract, with the initial symptoms of cold, fever, dry cough, fatigue, nasal congestion, sore throat, diarrhea to severe pneumonia, difficulty in breathing, and ends with the death of patient [4]. Patients suffered acute respiratory distress syndrome for about 7-10 days after the onset of COVID-19 due to rapid replication of the virus.
There are several strategies known to overcome viral infection; either blocking the receptor to prevent the entry of viruses, destroy the machinery i.e. prevention of replication, prevention of release or shredding, and activation of natural killer cells to kill the infected cells [5]. The treatment of patients varies based on disease severity. Several drugs have emerged for treatment, including nucleotide analogs (remdesivir) and anti-malarial (chloroquine), hydroxychloroquine). Protease inhibitor (lopinavir) and interferon-B have been under clinical trial, but are not recommended for treatment at this time [6]. SARS-CoV-2 is contagious, and there has not been any vaccine or effective treatment that has received approval [7].In this review, we highlight the pandemic potential and pathological indications of emerging coronavirus, and comprehensively and systematically summarize the up-to-date knowledge of the biological characteristics of 2019-nCoV, including characteristic symptoms, details of treatment, and current medical challenges to the World.
Symptoms
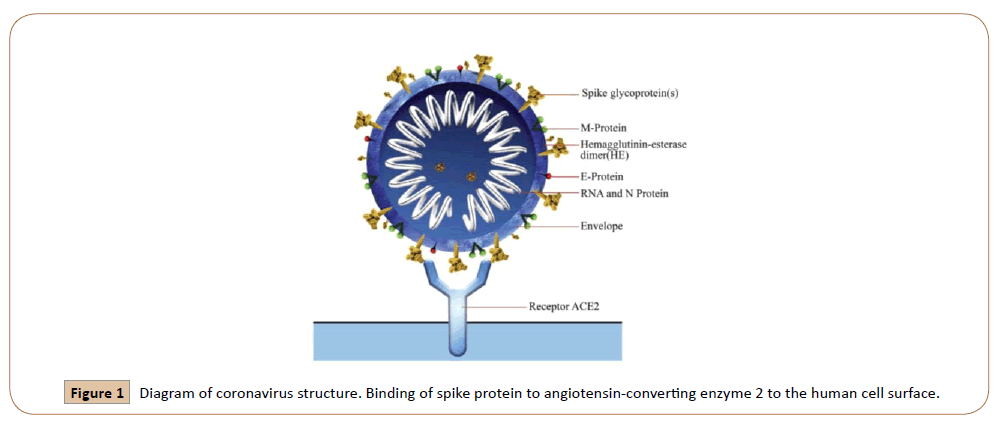
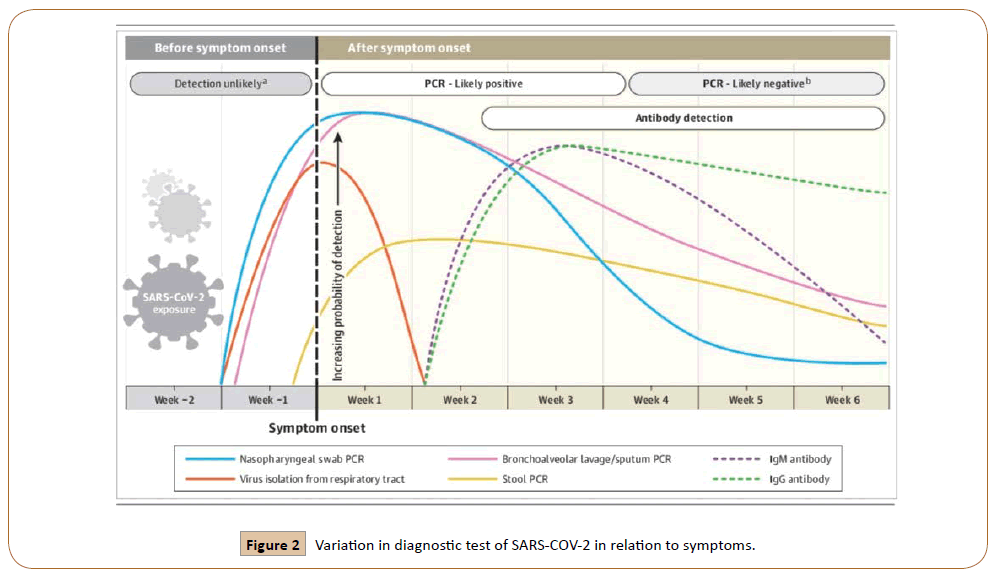
Coronavirus disease (COVID19) is an infectious disease and newly discovered coronavirus is the cause of it [8]. In the last two decades, several viruses emerge that are associated with the loss of human lives. Based on scientific studies, these animal viruses attained the capacity to cross-species and infected humans. The novel SARS-CoV-2 infection generally disturbs the air passageway and binds the hACE2 receptor on mucosal cells. When the spike protein binds with hACE2 results in different symptomatic outcomes (Figure 1) [9,10].Before the start of symptoms, the median incubation period is 4-5 days. 97% of symptomatic patients developed the symptoms within 11.5 days Omolo, C.A et al. [11]. The most common symptoms of coronavirus disease are fever, dry cough, and tiredness. There are some other symptoms from which people are less affected include aches and pains, nasal congestion, headache, sore throat, diarrhea, loss of taste or smell, rashes on the skin, discoloration of fingers, or toes [8]. By Jan 2, 2020, out of 41 patients admitted in hospital infected with COVID19 in Wuhan, China, 30 (73%) were men. Some about less than half had underlying disease including diabetes (20%), hypertension (15%), and cardiovascular disease (15%). Common symptoms of illness were fever (98%), Cough (76%), myalgia (44%). Less common symptoms were Sputum production (28%), headache (8%), hemoptysis (5%), and diarrhea (3%) [12]. COVID19 shows its sign in the form of disorder of the central or peripheral nervous system, acute cardiac, kidney, liver injury, coagulopathy, shock, and lymphopenia [11].Due to viral infection in the lungs, throat, and trachea, these become viral factories that cause damage to other cells. High temperature and feelings of malaise are the results of the response of the immune system to the virus and send the signal to release cytokines. Shortness of breath occurs when alveoli begin to fill with water. Sometimes these results are in the form of coughing with sputum, it is thick mucus consisted of killed lung cells [12,13]. In SARS-CoV infection, males are at higher risk of mortality than females. Imbalanced level of angiotensin-converting enzyme 2 (ACSE2) between males and females is the main factor in the sex-based difference in response to disease [14]. This first US case confers the respiratory symptoms first and it is followed by abdominal discomfort and self-limiting nausea, vomiting, and diarrhea [15]. Destruction of gastrointestinal bacteria leads to the above-mentioned symptoms of digestive system [13]. The symptomatic characteristics of SARS-COV-2 are similar to SARS-COV and MARS-COV. The first case in China indicates that the incubation period is between 2 to 7 days, however, the longest incubation period noticed was 12.5 days [11].Commonly COVID-19 is detected by RNA based RT-PCR test. RNA sample is taken from oropharyngeal swabs, sputum, nasopharyngeal aspirate, bronchoalveolar lavage, and tracheal aspirate. Rapid diagnose of infection is limited during initial stages because of the inapplicability of RT-PCR. The cause of this inapplicability is a low viral load, inappropriate sample, and undeveloped technology (Figure 2) [10].
Treatment
On the global outbreak of coronavirus disease (COVID-19), treatment options are limited. New therapeutic options are needed but it requires time. No approved treatment is specific for action against COVID-19. Different potential drugs are in use including antimalarial hydroxychloroquine, antiretrovirals lopinavir/ritonavir, and influenza drugs oseltamivir, remdesivir, and favipiravir [16,17]. Some specific treatments against coronavirus disease are under investigation and will be tested through trials. WHO is helping to accelerate researches [8]. With the continuous rise in the number of patients and death rates, more drugs and treatments are required for SARS-COV-2. On the base of target anti- coronavirus therapies are classified into two different categories. The one that targets the immune system and infected human cells. Other target the virus itself, the ability of the human innate immune system to gain the control of replication of virus and infection indicates the severity of disease [11].
General treatment for viral infection
Nutritional supplements: Vitamin A causes a reduction in the mortality in infectious diseases including measles, diarrheal disease, human immunodeficiency virus (HIV) infection, and malaria. Replication of SARS coronavirus is inhibited by the combination of zinc and pyrithione [18]. Zinc has antiviral effect for many respiratory viruses. Zinc treatment is performed for the inhibition of viral replication [19].
Allopathy medicines: Allopathic treatments include oxygen therapy, an intravenous fluid infusion that supports life. Recently, an Italian patient is treated by using the combination of lopinavir (200mg) and ritonavir (50mg) twice a day, the patient test was negative for COVID-19. However, some drugs are still under clinical trials.
Homeopathy: In homeopathy, arsenic is considered as effective or beneficial for disease including antiviral infection. The use of homeopathic medicines for COVID-19 is criticized by Thailand Medical News Jakkapong Watcharachaijunta. However, the research of Dr. Robert T shows that the Arsenicum album is effective to treat fever, runny nose, headache, sore throat. It is suggested that more research work is required to make the situation clear [20].
Herbal medicines: Treatments are based on plant extract without any side effects. The most important plants are Glycyrrhiza glabra, Allium cepa, Ocimum sanctum, Piper nigrum, Allium sativum, Curcuma longa. The root of Glycyrrhiza glabra has a good antiviral effect. Reports indicate that this plant is active against SARS, HIV, and SARS- associated coronaviruses [20]. Rheum Officinale, Polygonum multiflorum plant extract have active components that were found to inhibit the binding of SARS-COV(S) spike protein to ACE2 with IC50 [21].
Mechanism of action of vaccines
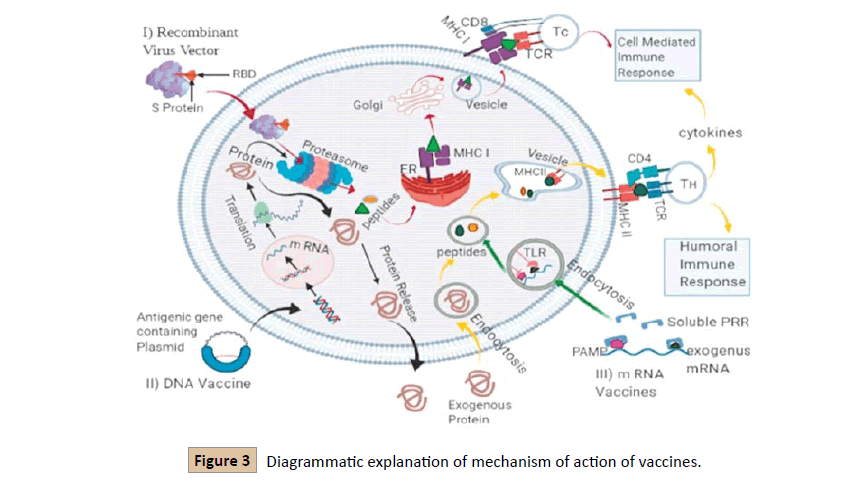
The vaccine is known to be the most effective measure against infections [22]. Humoral and cellular responses are necessary to fight against infection. For the designation of the vaccine, the full sequence of spike proteins of novel coronavirus requires or used for the prediction of B-cell and T- cell epitopes. The selection of best T-cell based on antigenicity Recombinant virus vector acts similarly as the androgenous antigen, after their processing in the proteasome, MHC I presents them to the CD8+Tc cells and cause Cell-Mediated Immune (CMI) response; II. Transcription and Translation of DNA occur in host cells. Synthesized protein move towards MHC class I pathway or released outside the cell for the action as exogenous antigen and presents to CD4+ TH cells and cause a humoral immune response and release cytokines by vTH cells leads to CMI response as well; III. PRR (Pathogen Recognition Receptor) recognize the mRNA endocytosis occurs and leads to the MHS class II pathway and cause a humoral immune response (Figure 3) [4].
Antiviral treatment
Antiviral drugs are used for viral infection. Researchers are working to develop an effective vaccine against COVID-19.
There are some antiviral drugs which are being developed and investigated to treat the mutated viral genomic disease of coronavirus.
Remdesivir: Remdesivir is a phosphoramidite prodrug of an adenine derivative that has a chemical structure like Tenofovir alafenamide [7]. Remdesivir is the first rank potential drug in the treatment of COVID-19 [23]. Remdesivir is active against a diverse range of coronaviruses; a recent study suggests that it is effective against COVID-19 [7]. Remdesivir stops the viral replication by inhibiting the viral enzyme (RNA-dependent RNA polymerase). This has enough potential to use as a therapeutic drug against COV in the future. It is the limited and high cost so lt becomes a challenge, there is a need to overcome this issue [24].
Chloroquine / Hydroxychloroquine, azithromycin: Chloroquine has been used for prophylaxis and treatment of malaria. Now chloroquine becomes ineffective for the treatment of malaria caused by P. falciparum. Apart from malaria, it is used to treat rheumatoid arthritis and lupus erythematosus [5]. However, both drugs have certain side effects such as worsening vision, nausea, digestive disorder, and leads to heart failure. A man and his wife were in a critical situation after taking chloroquine [13]. Azithromycin was effective against Ebola viruses. It has the potential to prevent severe respiratory tract infections in children [25]. Chloroquine and azithromycin combination show benefits but this may increase the risk and leads to death [26].
Cinanserins: Chymotrypsin-like and papain-like protease is coronavirus encoded protein. They have an essential role in coronavirus replication and inhibit the host immune response. Cinanserin inhibits the chymotrypsin-like (3C-like) proteases and inhibits the replication of SARS-COV [18].
Favipiravir: Favipiravir is an antiviral agent that selectively acts on the RNA dependent RNA polymerase (RdRP) of RNA viruses. It was synthesized by modifying pyrazine analog. In China patients of COVID-19 were recovered by using a combination of the existing antiviral drug (for SARS, Ebola, and AIDS) like Favipiravir [5]. After converted into an active form that is phosphoribosylated form. It is recognized as a substrate of viral RNA polymerase in many viruses [25].
Arbidol: Arbidol is a non-nucleoside antiviral and immunomodulatory drug for the treatment of influenza [7]. In China and Russia, it is used as an anti-influenza drug. Arbidol mesylate has anti- SARS-COV-2 effects [21]. No side effect was observed after treating patients with arbidol and no viral load was detected. Patients taking arbidol have shorter positive RNA test [5].
Amantadine: Amantadine is highly lipophilic and easily crosses the lysosome membrane where it accumulates and acts on higher micromolecular concentration. Amantadine inhibits the replication of influenza A at lower micromolecular concentration. Amantadine acts as a lysosomotropic substance and easily crosses the SARS-COV-2 lysosome membrane and accumulates here, lower the pH of lysosome thus inhibit the protease activity. Clinical studies will be needed to check the efficiency of amantadine against COVID-19 infection [27].
Lopinavir /Ritonavir: These are protease inhibitors and used in HIV treatment. They show antiviral activity in SARS-COV and MERS-COV. Ritonavir increases the half-life of lopinavir [23]. In China trials were performed, they show effectiveness for the treatment of adults suffering from SARS-COV-2 with oral use of Lopinavir and Ritonavir. However, further studies needed to show the results [28].
Other antivirals: Oseltamivir is neuraminidase is used to treat influenza A and influenza B viruses. It is an inhibitor of neuraminidase enzyme that is involved in the cleavage of sialic acid. Sialic acid is the component of glycoprotein. Few of the combinational drugs such as ASC09F and Ritonavir are evaluated with Oseltamivir. These are under clinical trial [28]. Ribavirin is approved for treating respiratory syncytial virus (RSV) and the hepatitis C virus (HCV). It also shows a positive result for SARS and MERS but it has side effects such as anemia. It is not clear whether it is effective against SARS-COV-2 or not [7].
Therapies
Corticosteroids: Corticosteroids are used for their antiinflammatory effects in patients that face respiratory infections. Studies have shown that the use of corticosteroids in patients infected with COVID-19 has side effects and it is associated with delayed viral clearance, higher risk of secondary infections, and increased risk of mortality. It is not used for the treatment of COVID-19 infection but it may be required to treat other conditions that may accompany it [6].
Convalescent plasma/Immunoglobulin therapy: It has been used to improve the survival rate of infected patients. In Convalescent plasma therapy immunoglobulin antibodies in the plasma of patients recovering from viral infection suppress viremia [25]. Clearance and restriction of viral entry are done by virus-specific antibodies through viral clearance [29]. In China five critically ill patients show improvement, after receiving plasma their body temperature move toward normal state within 3 days (four out of five patients) and three out of five patients discharged from hospital and they were in normal condition at 37 days of therapy. US FDA approved it for the patients who were suffering from COVID-19. However, there are certain risks and ethical issues related to this therapy. Risk of thrombotic events, lack of highquality research, and selection of donor with high neutralizing antibodies titer [1].
Inhaled nitric oxide: Nitric oxide is used for smooth muscle relaxation, immune responses, and antimicrobial activities. It is widely used for pulmonary vasodilation. Previously INO therapy is used for SARS patients; it is followed by recovery with improved atrial oxygenation. This therapy is under clinical trial for COVID-19 infected patients [30].
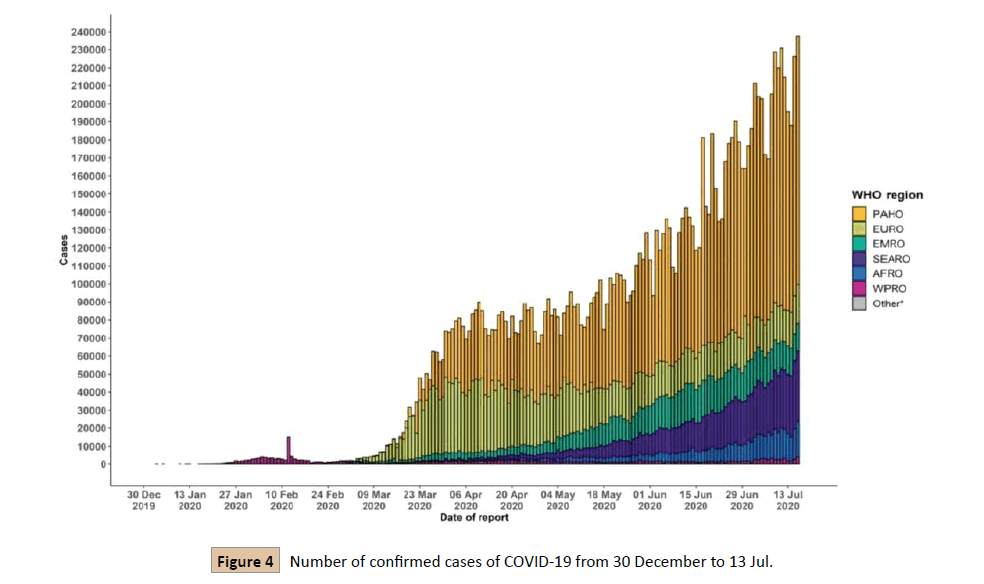
Interferon-based therapy: Interferons play a role in the regulation of the innate immune system that on the other way plays a vital role in killing the viruses by regulating the gene transcription. Interferon therapy is useful for treating COVID-19 [7]. Interferons are used to treat skin diseases. The interferonalpha and beta are of several types I, II, and III IFNs [23]. In children, the lower mortality rate is due to the rapid production of interferons after infection. It can stimulate the immune system by directly suppressing the SARS-COV-2. Interferon therapy may be effective against SARA-COV-2 [7]. Coronavirus is an agent that causes fatal infection or illness [31]. In the past, many different COVs have been identified that became the cause of infection in humans and animals. In November 2002 SARS-COV identified in china that spread in 37 countries with 8000 reported cases and 800 deaths. This SARS was originated from horseshoe bat. At that time no vaccine was available but preventive measures were implemented such as social distancing, quarantine, travel restriction, and patient isolation.In 2012, a COV was emerged in Saudi Arabia originated from camel, which infected 2494 people, and 858 deaths were reported. In late December 2019, novel coronavirus have evolved and connected with the seafood market in Wuhan, Hubei Province, China [28]. Till 17 July 13,616,593 cases were reported (Figure 4) [8].
Critical patients reported in china with COVID-19 infection are older and carry other comorbidities, such as hypertension, diabetes. Older patients account for more than 80% for death. Incubation of patient infected with COVID-19 increase the risk of viral transmission to health care worker, to avoid this transmission the most skilled operator should perform the task with personal protective equipment (PPE). To reduce exposure assistant should be limited.
Bag mask ventilation produces aerosol so, it should be minimized by prolonged pre -oxygenation. The ventilator-induced injury must be avoided. ICU capacity must be balanced [32]. In states such as Massachusetts and Pennsylvania and some European countries, residents of nursing homes account for 50% of deaths. Nursing homes are in lockdown, they even unable to see their family members and participate in normal activities. Viruses spread in nursing homes through staff in the absence of sufficient PPE. This sector will need PPE and need training that supports them to control infection. Social and medical care will become the need for residents of the nursing home to recover from COVID-19 [33].Radiological evaluations have roll in diagnosis and management, but the final diagnosis of COVID-19 is based on RTPCR. This is the clinical method to confirm the COVID-19 infection. The false-negative results and inapplicability during the initial phases limit the rapid diagnosis. The causes for low effectiveness are inappropriate sample, low viral load, variation in diagnosis in different kits, and underdeveloped technology [10].COVID-19 is a life-threatening pandemic; therefore the provision of solution and cure becomes the urgent need. Clinical studies are going on and some drugs show encouraging results [11]. Drug discovery is a challenging process. Virus drug development becomes advanced during the past few years. It is challenging because viruses are clever and become resistant to available drugs quickly [5]. Recently there are no active antiviral drugs available to treat SARS-COV-2. Many treatment are used on a trial basis or limited data [6].In vaccine manufacturing, insurance of safety and efficacy are the main objectives [23]. Repetition of vaccine is useful for acquiring the immunity against infectious diseases [28]. Vaccines are prepared from dead microorganisms. Vaccine preparation requires a large number of participants, evaluation of long term implications on human beings, and also identifies the toxicity. One of the main limitations observed is that no long term protocol has been undertaken for the safety and efficacy of drugs proposed for SARS-COV-2, for this reason, long term studies require for drug development [11].
Recent Challenges to the World
Duty of care
Every patient is owed the best possible care and treatment available in the circumstances. Even when resources need to be rationed during a crisis, health care professionals and frontline workers have a duty of care to promote their patients’ welfare within available resources. Health care professionals and frontline workers are also owed a duty of care. In this regard, appropriate PPE for health care professionals and frontline workers should be provided to promote their safety and well-being. This is a benefit to them but also to the whole of society by ensuring that they are available to support the clinical response for as long as possible [34].
Protection of the community
Appropriate IPC should be in place, respected, and enforced. Such actions protect patients, health care professionals, and the community. During a pandemic, the focus should be on both clinical care for patients and the promotion of public health [34].
Confidentiality
All communications between patient and clinician must remain confidential except in the case of compelling public health concerns (e.g. contact tracing and surveillance etc.) or other accepted justifications for breach of confidentiality. Private individual information must be kept secure unless it is a justified breach. Older age has been reported as a risk factor for increased mortality in those affected by COVID-19. Other risk factors that have been reported are smoking, diabetes, hypertension, cerebrovascular disease, cancer, and chronic lung disease. Since older people are often affected by these conditions as well, they are potentially at the highest risk for fatality. Those with frailty are one of the most vulnerable populations [34].
Health and social systems
Implementation of guidelines and policies for the care of people with COVID-19 should, as far as possible, build on community and hospital health services and other sectors of society, including the social and private sectors. But the problem is this policy implementation is not possible in every region of the world.
Information and communication
The provision of clear, consistent information about COVID-19, including how it spreads and how to prevent transmission, is a key part of implementing the guidance. Such information is not tailored to different groups, available in local languages, expressed in simple, clear text and attractive images that speak to local populations. These images should include older and younger people, people from different ethnic groups, and people with disabilities. Real-life images are the preferable source but its access is very limited.
Lack of Control practices
Information alone is not enough to ensure good infection control practices and adherence to recommended measures and behavior to prevent transmission. Several factors affect people’s ability to follow recommended guidance, including their perception of the risk of becoming infected, their beliefs about COVID-19 and COVID-19 care, their attitudes and beliefs about the effectiveness of recommendations, and the extent to which recommendations are practical and feasible in their living environment These factors may also change over time like obtaining food, water and medicines. There is lack of control practices of mentioned factors [35].
Acknowledgement
The Authors would like to acknowledge the Institute of Energy and Environmental Engineering, University of the Punjab, Lahore, Pakistan.
Authors Contribution
All authors contributed equally in designing, data collection, assimilation and writing of this manuscript and the final version was read and approved by all authors.
Conflict of Interest
The authors declare no conflict of interest.
References
- Tobaiqy M, Qashqary M, Al-Dahery, Mujallad A, Hershan AA, et al. (2020) Therapeutic managment of pateints with COVID-19. Infection Prevention in Practice 2: 100061.
- Landry MD, Geddes L, Moseman AP, Lefler JP, Raman SR, et al. (2020) Early reflection on the global impact of COVID-19, and implication of physiotherapy. Physiotherapy 107: PA1-A3.
- Panyod S, Ho CT, Sheen LY (2020) Dietary therapy and herbal medicine for COVID-19 prevention. J Tradit Complement Med 10: 420-427.
- Pandey SC, Pande V, Sati D, Upreti S, Samant M (2020) Vaccination strategies to combat novel corona virus SARS-COV-2. Life Sci 256: 117956.
- Akhtar MJ (2020) COVID19 inhibitors: A prospective therapeutics. Bioorg Chem 101: 104027.
- Mehta N, Mazer-Amirshahi M, Alkindi N, Pourmand A (2020) Pharmacotherapy in COVID-19: A narrative review for emergency providers. Am J Emerg Med 38: 1488-1493.
- Lotfi M, Hamblin MR, Rezaei N (2020) COVID-19: Transmission, prevention, and potential therapeutic opportunities. Clin Chim Acta 508: 254-266.
- WHO (2020) World Health Organization.
- Abdulmohsen H, Al-Rohaimi, Al Otaibi F (2020) Novel SARS-COV-2 outbreak and COVID19 disease. Genes Dis.
- Abbasi-Oshaghi E, Mirzaei F, Farahani F, Khodadadi I, Tayebinia H (2020) Diagnosis and treatment of coronavirus diseases 2019 ( COVID-19). Int J Surg 79: 143-153.
- Omolo AC, Soni N, Fasiku VO, Mackraj I, Govender T (2020) Updates on therapeutic approaches and emerging therapies for SARS-COV-2 virus. Eur J Pharmacol 883: 173348.
- Huang C, Wang Y, Li X, Ren L, Zhao J, et al. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet 395: 497-506.
- El-Aziz TMA, Stockand JD (2020) Recent progress and challenges in drug development against COVID-19 coronavirus (SARS-COV-2). Infect Genet Evol 83:104327.
- Shi D, Wu W, Wang Q, Xu K, Xie J, et al. (2020).Clinical characteristics and factors associated with long-term viral excretion in patients with SARS-COV-2 infection. J Infect Dis 222:910-918.
- Lupia T, Scabini S, Pinna SM, Perri GD, Rosa FGD, et al. (2020) 2019 novel coronavirus (2019-nCOV) outbreak. J Glob Antimicrob Resist 21: 22-27.
- Sethuraman N, Jeremiah SS, Ryo A (2020) Interpreting Diagnostic Test for SARS-COV-2. JAMA 323: 2249-2251.
- Pilkington V, Pepperrell T, Hill A (2020) A review of the safety of favipiravir- a potential treatment in the COVID-19 pandemic? J Virus Erad 6: 45-51.
- Khodadadi E, Maroufi P, Khodadadi A, Esposito I, Khudaverdi G, et al. (2020) Study of combining virtual screening and antiviral treatment of Sars-COV-2 (COVID-19). Microb Pathog 146: 104241.
- Read AS, Obeid S, Ahlenstiel C, Ahlenstiel G (2019) The role of a Zinc in Antiviral Immunity. Adv Nutr 10: 696-710.
- Ali I, Alharbi OML (2020) COVID-19: Disease, management, treatment, and social impact. Sci Total Environ 728: 138861.
- Mirzaie A, Halaji M, Dehkordi FS, Ranjbar R, Noorbazargan H (2020) A narrative literature review on traditional medicine options for treatment of corona virus disease 2019 (COVID-19). Complementary Therapies in Clinical Practice 40:101214.
- Chen HW, Huang CY, Lin SY, Fang ZS, Hsu CH, et al. (2016) Synthetic virus-like particles prepared via protein corona formation enable effective vaccination in an avian model of coronavirus infection. Biomaterials 106:111-118.
- Ortiz-Prado E, Simbana-Rivera K, Gomez-Barreno L, Rubio-Neira M, Guaman LP, et al. (2020) Clinical, molecular, and epidemiological characterization of the SARS-COV-2 virus and thev coronavirus disease 2019 (COVID-19). Diagn Microbiol Infect Dis 98: 115094.
- Venkatasubbaiah M, Reddy PD, Satyanarayana SV (2020) Literature-based review of the drugs used for the treatment of COVID-19. Current Medicine Research and Practice 10: 100-109.
- Jean S-S, Lee P-I, Hsueh P-R (2020) Treatment options for COVID-19: The reality and challenges. J Microbiol Immunol Infect 53: 436-443.
- Vouri M S, Thai NT, Winterstein A G (2020) An evaluation of co-use of chloroquine or hydroxychloroquine plus azithromycin on cardiac outcomes. Res Social Adm Pharm.
- Smieszek SP, Przychodzen BP, Polymeropoulos MH (2020) Amantadine disrupt lysosomal gene expression:A hypothesis for Covid 19 treatment. Int J Antimicrob Agents 55:106004.
- Poolanda V, Thatikonda S, Godugu C (2020) The current understanding and potential therapeutic options to combat. Life Sci 254: 117765.
- Chan W, He B, Wang X, He M-L (2020) Current status and challenges of antiviral therapies. Genes Dis.
- Zang J, Xie B, Hashimoto K (2020) Current status of potential therapeutic candidates for the COVID-19 crisis. Brain Behav Immun 87: 59-73.
- Rowan NJ, Laffey JG (2020) Challenges and solution for addressing critical shortage of supply chain for personal and protective equipment (PPE) arising from Coronavirus disease ( COVID 19) pandemic. Sci Total Environ 725: 138532.
- Phua J, Weng L, Ling L, Egi M, Lim CM, et al. (2020) Intensive care management of coronavirus disease 2019 (COVID-19). The Lancet Respiratory Medicine 8: 506-517.
- Grabowski DC, Mor V (2020) Nursing Homes Care in Crisis in the Wake of COVID-19. JAMA 324:23-24.
- Clinical management of COVD-19 (2020) WHO interim guidance.
- Home care for patients with suspected or confirmed COVID-19 and management of their contacts (2020) WHO interim guidance.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences