The Budget Impact of the DISCERN ™ Diagnostic Test for Alzheimer's Disease in the United States
Lauren Fusfeld1*, Frederick Huie2, Scott Howell3, Alyssa McVey1 and Thomas F Goss1
3Semler Scientific, Santa Clara, USA
Received date: April 27, 2023, Manuscript No. IPJPM-23-16517; Editor assigned date: May 01, 2023, PreQC No. IPJPM-23-16517 (PQ); Reviewed date: May 15, 2023, QC No. IPJPM-23-16517; Revised date: May 22, 2023, Manuscript No. IPJPM-23-16517 (R); Published date: May 29, 2023, DOI: 10.36648/2572-5483.8.2.192
Citation: Fusfeld L, Huie F, Howell S, McVey A, Goss TF (2023) The Budget Impact of the DISCERNTM Diagnostic Test for Alzheimer’s Disease in the United States. J Prev Med Vol.8 No.2: 192.
Abstract
Background: This study evaluates the budget impact from a payer perspective of using DISCERNTM in the diagnosis of patients with symptoms of dementia evaluated for Alzheimer’s Disease (AD).
Study Design: A decision-tree framework and mathematical model were used to estimate clinical and cost implications of testing with DISCERNTM.
Methods: An excel-based model with a three-year horizon was developed to assess the budget impact of DISCERNTM compared with the Current Diagnostic Pathway (CDP) to diagnose AD in a Medicare Advantage plan with 1 M beneficiaries. A targeted literature review was conducted to identify values for model parameters, which were verified through consultation with clinicians experienced in the diagnosis and management of AD. A one-way sensitivity analysis, with model parameters varied by ± 10%, was conducted to assess relative effect of key clinical and cost parameters.
Results: DISCERNTM is estimated to decrease total costs by $4.75 M over three years, which equates to approximately $63.11 net savings per test per year for a cohort followed over three years. While the cost of diagnosis with DISCERNTM is higher than the cost of diagnosis with CDP modalities, the overall costs associated with the CDP are larger due to the longer time needed to receive a diagnosis as well as the higher number of patients who receive a misdiagnosis and require extra care.
Conclusion: DISCERNTM improves the rate of accurate, definitive diagnoses of AD earlier in the disease and may have the potential to generate savings for Medicare Advantage.
Keywords
Alzheimer’s disease; Budget; Dementia; Diagnosis
Introduction
Alzheimer’s Disease (AD) is a degenerative brain disease characterized by memory loss and cognitive decline [1,2]. Mild AD symptoms include difficulty recalling recent events and completing normal cognitive tasks. Patients with moderate AD become dependent on caretakers for some Activities of Daily Livings (ADLs) and patients with severe AD experience motor and balance impairments and require a full-time caretaker to help with most ADLs [3].
AD occurs primarily in individuals over the age of 65 [1,3,4]. In 2022, the estimated prevalence of Americans aged ≥ 65 with AD dementia was 6.5 million, a number projected to rise to 13.8 million by 2060 [5]. The economic burden of AD is substantial, with total payments for individuals with AD and other dementias by patients and health insurers in the US estimated at $321 billion in 2022; of which Medicare and Medicaid are expected to cover approximately $206 billion (64%). Growth in the number of patients diagnosed with AD is expected to increase US health care spending to approximately $1 trillion by 2050.
The standard defined by the US National Institutes of Health (NIH) for diagnosing AD requires an autopsy-confirmed presence of beta-amyloid and tau protein deposition in patients with symptoms of dementia [6]. The Current Diagnostic Pathway (CDP) includes multiple testing modalities, such as Magnetic Resonance Imaging (MRI), Computed Tomography (CT), Positive Emission Tomography (PET), Cerebrospinal Fluid (CSF) biomarker tests and blood tests, which are used variably among providers and are unable to diagnose AD definitively and distinguish it from other forms of dementia [7,8]. Reported sensitivity and specificity of currently available diagnostics are between 70.9%– 87.3% and 44.3%–70.8%, respectively [9]. Additionally, access to diagnostics that detect amyloid and tau protein plaques is limited by the diagnostics’ cost and limited coverage by health insurers [10,11].
DISCERNTM was developed to distinguish AD accurately from other forms of dementia, even in patients recently diagnosed with dementia and those with mixed dementia. DISCERNTM comprises three assays that assess several critical factors related to AD that regulate memory, the formation of synaptic connections among neurons, the levels of amyloid plaques and the levels of neurofibrillary tangles in the brain. These assays accurately produce an objective report indicating whether the patient is “positive” or “negative” for AD, allowing physicians to optimize medications prescribed for cognitive impairment indicated in AD. DISCERNTM has been validated extensively using autopsy-confirmed AD and has demonstrated significant improvements over clinical diagnosis alone in people recently diagnosed with dementia, achieving sensitivity and specificity >95% for the diagnosis of AD [12-15].
Materials and Methods
Model overview
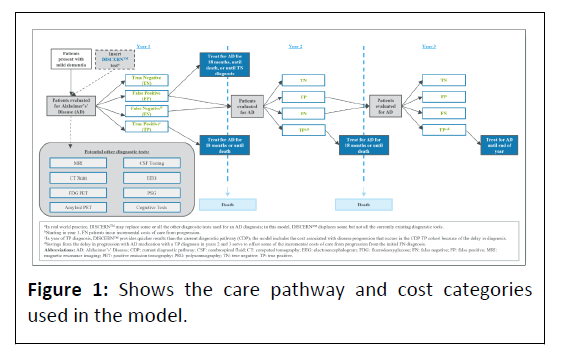
An excel-based model was developed in accordance with the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) good practices for budget impact analyses [16]. The three-year model estimates the budget impact to a Medicare Advantage plan of 1 Million (M) beneficiaries of using DISCERNTM in the diagnosis of patients with symptoms of dementia evaluated for AD compared with the CDP. The model assesses direct medical costs/savings of patients based on the following:
Evaluation/Diagnosis: The costs of diagnosis using DISCERNTM and the CDP.
False Negative (FN) diagnosis: The incremental cost of care avoidable with a correct AD diagnosis and appropriately directed medication. The cost was based on the weighted average of the distribution of mild/moderate/severe AD direct costs by year and the percentage of patients’ annual progression that could be avoided with a correct diagnosis.
True Positive (TP) diagnosis: 1) AD medication costs following a TP diagnosis. 2) The cost from a later TP diagnosis with the CDP versus DISCERNTM given DISCERNTM’s ability to provide quicker results than the CDP in the year of diagnosis. 3) The savings from the delay in AD progression due to appropriate AD medication for patients who are correctly diagnosed after a FN diagnosis. The patient’s stage of AD impacts the magnitude of this benefit and determines whether the savings amount is a complete or partial offset of the extra cost of care associated with the initial FN diagnosis. If a patient is still in the mild stage, the TP diagnosis offset 100% of the incremental cost of FNrelated care. However, the savings from a TP diagnosis declines as a patient progresses in the model, with 88% and 75% of the incremental cost of FN-related care offset in moderate and severe AD, respectively [17,18].
False Positive (FP) diagnosis: Cost of AD medication for patients who do not have AD. All patients with a FP diagnosis were assumed to receive treatment for mild dementia.
True Negative (TN) diagnosis: No cost (aside from the initial diagnostic cost). Treatment costs of patients with a TN diagnosis were not included in the model Figure 1.
Data and assumptions
A focused literature review was conducted to identify model inputs, which were verified through consultation with clinicians experienced in the diagnosis and management of AD (SH and FH) to ensure the values reflected real-world experience. Given current limitations in available longitudinal testing and outcomes data, several assumptions were made to simplify the model. First, patients in the model are evaluated for AD at the beginning of the year and are evaluated each year they are alive until they receive the correct diagnosis or until the end of the model timeframe. Additionally, patients who receive a correct diagnosis (i.e., a TP or TN) do not receive any further diagnostic evaluation after the diagnosis is established. Second, the frequency of testing used for the evaluation of AD decreases by 20% per year across all testing modalities (aside from DISCERNTM) in years 2 and 3 for patients who have been misclassified (i.e., received FP or FN). Third, patients who die within the time frame of the model are assumed to die at the end of the year. Fourth, the model assumes all patients with an AD diagnosis will receive treatment for AD, while patients with a negative AD diagnosis will not receive any AD treatment. Finally, while patients who receive a negative diagnosis may incur treatment costs for another form of dementia, these costs are not included in this model given the breadth of possible non-AD diagnoses.
Model parameter estimates
Epidemiology: The number of new candidates eligible for testing each year was estimated from the percentage of Medicare Advantage beneficiaries, the incidence of dementia in patients aged ≥ 65 and the percentage distribution of mild, moderate and severe dementia among these patients (Appendix 1) [4,19,20].
Distribution of patients receiving diagnostics: A recent survey of primary care physicians, neurologists and geriatricians was used to provide utilization rates of CT scans, MRIs, CSF biomarker testing and amyloid PET scans in the CDP (Appendix 1) [8]. In the DISCERNTM arm, all patients receive DISCERNTM, along with some of the currently available diagnostic tools according to clinicians’ expected use once DISCERNTM becomes available.
Diagnostic performance characteristics: The CDP performance characteristics used in the model represent the entire diagnostic workup (Appendix 1) [9,21]. The performance characteristics of DISCERNTM from validation studies were applied to the use of DISCERNTM in conjunction with other diagnostic tools in the model [12,13,22,23].
Average time to an AD diagnosis: The average time to diagnosis of AD using the CDP was based on the reported time to confirmation of a diagnosis from the initial office visit for a “usual care” dementia cohort from the New England Veteran Healthcare System (Appendix 1) [24]. The average time to diagnosis for DISCERNTM was derived from validation studies [12,13,22,23].
Duration of AD treatment: The duration of AD treatment in the model was an average obtained from reported treatment durations of donepezil across several studies (Appendix 1) [25-28]. Donepezil was selected on the assumption that most patients with mild AD will receive this drug at the start of treatment. Additionally, AD medication use was assumed to stop once a patient transitions to severe AD, as symptom-modifying treatments have little impact on disease progression in this stage [28,29].
Delay in AD progression due to medication for the treatment of symptoms: The duration of delay in progression of AD attributable to medication use is presented in Appendix 1. Studies examining the long-term effects of cholinesterase inhibitors like donepezil indicate that treatment produces an average delay in cognitive decline in AD patients of 9 months–12 months [30]. Thus, the extra care needed for untreated AD patients who progress because of a FN diagnosis was calculated by taking 75% of the direct medical costs of AD from diagnosis through month nine post-diagnosis.
Progression rates between stages of AD with and without medication: While current medications do not impact the underlying physiology of AD, early and appropriate utilization of these medications enhances cognitive function [31]. The rates of progression between the stages of AD were derived from a cost effectiveness study of donepezil for the treatment of mild or moderate AD and data from a 24-week randomized clinical trial in which the enhancement of cognitive function due to donepezil treatment was projected to slow disease progression over time. The patient distribution across stages of AD over three years is presented in Appendix 1.
Cost inputs: The model assesses direct medical costs paid by a Medicare Advantage plan for AD patients, including medications, hospitalizations, emergency room visits, outpatient visits and neuropsychological assessments. Annual direct costs for mild, moderate and severe stages of AD were derived from a prospective cohort study [32]. These costs, originally reported in 2017 USD, were inflated to 2022 USD using the US Bureau of Labor Statistics consumer price index data for Medical Care [33]. Costs of different diagnostics, including cost of diagnostic tools, laboratory analyses, office visits and neuropsychological testing, were taken from 2022 Medicare Physician and Clinical Laboratory Fee Schedules and Coverage Policies [11,34-38]. The cost of DISCERNTM is a weighted average of two proprietary laboratory analyses codes: Code 0206U, which represents the two assays performed on every sample and code 0207U, which represents the third assay performed following discordance or indeterminate results (approximately 20% of samples) [36].
Finally, individual AD medication costs were obtained from PriceRx and an average annual cost was calculated based on reported prescribing rates of physicians in a multi-country survey and the distribution of mild, moderate and severe AD by year [29,39]. To avoid double counting costs, diagnostic and medication costs included directly in the model were subtracted from the published estimates of the annual cost of a given AD stage, where appropriate. Costs inputs are presented in Table 1.
| Annual direct AD costs by stage | ||||||||
| Stage | Total 2022 per patient costa | Source | ||||||
| Mild AD | $17,527.10 | Robinson, et al., [32] | ||||||
| Moderate AD | $20,752.09 | |||||||
| Severe AD | $26,645.69 | |||||||
| Costs for diagnostic modalities | ||||||||
| Diagnostic modality | Average cost to payer | CPT Codesb | APC Codesb | Sources | ||||
| CT scan | $219.90 | 70450 | 5522 | EncoderPro [38] CMS April 2022 OPPS payment Addendum [36] CMS April 2022 ASC Addendum [35] 2018 Medicare National HCPCS Aggregate Report [37] Evicore 2020 Head Imaging Policy [11] |
||||
| MRI | $490.33 | 70551 70553 |
5523 5572 | |||||
| CSF testing | $676.64 | 62270+ 83520 (x3) | 5442 | |||||
| Amyloid PET | $1,511.34 | 78814 78811 | 5594 | |||||
| FDG PET | $1,581.46 | 78608 | 5594 | |||||
| EEG | $130.47 | 95717 95719 | - | |||||
| PSG | $1,016.51 | 95810 | 5724 | |||||
| DISCERNTMd | $2,317.20 | 0206U 0207U | - | |||||
| Office visit | $131.64 | 99241 99242 99243 99244 99245 | - | |||||
| Neuropsyc-hological testsc | $849.67 | 96116 96132 96133 96136 96138 96139 | - | American Psychological Association Service, Inc, 2019 Psychological and Neuropsychological Testing: Billing and Coding Guide, Addendum [34] | ||||
| Annual costs of AD Medications by stagee | ||||||||
| Stage | AWAC | Sources | ||||||
| Mild AD | $625.56 | Podhorna, et al., 2020 [29] Price Rx [39] | ||||||
| Moderate and severe ADf | $752.23 | |||||||
| Note: a) 2017 costs were updated to annual rates and inflated to 2022 USD using CPI data for Medical Care from the Bureau of Labor Statistics. b) In instances where there is more than one CPT or APC code, an average was taken. c) Neuropsychological testing costs were informed by the American Psychological Association's 2019 Psychological and Neuropsychological Testing: Billing and Coding Guide. The guide provided three scenarios of how to bill for these services and the average of the three scenarios was taken to obtain the total average cost. d) To calculate the cost of DISCERNTM, it was assumed that 100% of patients receiving DISCERNTM are billed using code 0206U and 20% of patients are billed using both 0206U and 0207U. e) Drug regimens considered were the following: Donepezil (Aricept) monotherapy, rivastigmine (Exelon) patch monotherapy, rivastigmine (Exelon) oral monotherapy, memantine monotherapy, galantamine (Razadyne) monotherapy, donepezil + Namenda (memantine), donepezil + galantamine. Costs of generics were used. f) It was assumed that moderate and severe AD have the same medication cost. |
||||||||
Table 1: Total 2022 Cost inputs.
Abbreviations: AD: Alzheimer’s Disease; APC: Ambulatory Payment Classifications; AWAC: Average Wholesale Acquisition Cost; CDP: Current Diagnostic Pathway; CSF: Cerebrospinal Fluid; CPT: Current Procedural Terminology; CT: Computed Tomography; EEG: Electroencephalogram; FDG: Fluorodeoxyglucose; MRI: Magnetic Resonance Imaging; PET: Positive Emission Tomography; PSG: Polysomnography
Sensitivity analysis
One-way sensitivity analyses were conducted to assess the effect on three-year savings of varying model parameters by ± 10% in relative terms.
Results
Base case analysis
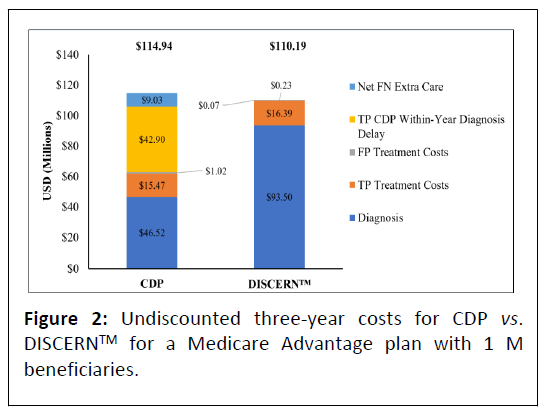
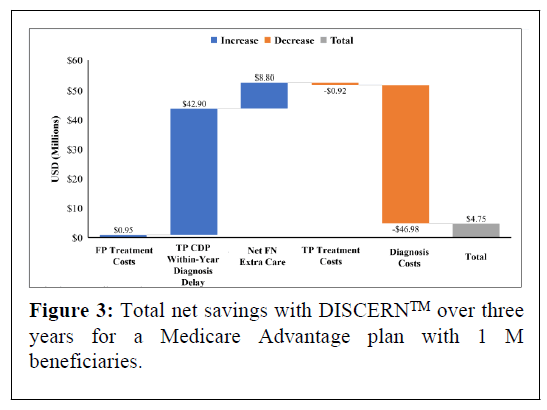
In the model 24,602 beneficiaries aged ≥ 65 with mild dementia are evaluated for AD, of whom 17,222 (70%) have AD. In the base scenario, overall undiscounted savings with DISCERNTM are estimated at $4.75 M over three years, which equates to approximately $63.11 net savings per test per year for a cohort followed over three years. Figure 2 presents undiscounted costs associated with the CDP versus the DISCERNTM testing arm (which includes reduced use of currently available diagnostic modalities) over three years. Figure 3 presents net savings associated with using DISCERNTM over three years. While the cost of diagnosis with the DISCERNTM arm is higher than with the CDP over three years, the overall costs associated with the CDP are greater due the delay in receiving a TP diagnosis and the extra cost of care required for FN patients. Most cost savings are realized in the TP and FN categories (Figures 2 and 3).
Abbreviations: CDP: Current Diagnostic Pathway; FN: False Negative; TP: True Positive; USD: United States Dollars.
Abbreviations: CDP: Current Diagnostic Pathway; FN: False Negative; TP: True Positive; USD: United States Dollars.
Sensitivity analysis
Results from the one-way sensitivity analysis are shown in Appendix Figure 2. The parameters with the greatest impact on lowering savings in the model are the following: Reduced sensitivity of DISCERNTM, improved sensitivity of the CDP, a reduction in the percentage of disease progression that is avoided with AD treatment, a reduction in the total annual costs of mild AD and a reduction in the percentage of mild dementia caused by AD. The absolute values of the changes in savings related to the top five inputs range from $4.95 M to 8.01 M (Appendix Figure 2).
Discussion
Discussion The need for an AD diagnostic test with better performance characteristics than the CDP is well established [8,40]. Beyond tests that can identify certain treatable causes of dementia symptoms (e.g., hypothyroidism, vitamin B12 deficiency, Lyme disease) and, hence; can rule out AD, no single test can definitively diagnose AD [7]. Additionally, the utilization of tests varies widely among clinicians, resulting in a lack of a true “standard of care” for AD diagnosis. Misdiagnosis also has cost implications; for example, one retrospective analysis of patients with vascular dementia showed that a misdiagnosis of AD resulted in higher direct costs than matched counterparts [41]. An accurate, definitive AD diagnostic tool could provide prompt access to current treatments as well as enable enrolment in clinical trials for promising new therapies [8]. The need for an improved AD diagnostic tool is especially important given projections of escalating costs of AD care as the population ages [5,40,42,43].
As DISCERNTM comprises multiple assays that yield superior sensitivity and specificity compared with the CDP [9], more patients receive a correct diagnosis with DISCERNTM, which allows for the use of appropriate medication earlier to delay progression to higher cost stages of AD. While the upfront costs of diagnosis in the DISCERNTM arm are larger than the cost of diagnosis with the CDP, overall net savings are realized due to the delay in receiving a TP diagnosis with the CDP and a higher proportion of patients misdiagnosed with CDP testing, as FN patients incur incremental costs associated with AD progression before they receive a correct AD diagnosis in the model. The benefit of a timely TP diagnosis is due to a reduction in the need for additional medical assistance, including institutional care, allowing clinicians to initiate appropriate treatment at an earlier stage. Given that institutional care is particularly costly, any minor delay to institutional care would have a meaningful impact on offsetting costs of the disease [24].
Despite its potential economic benefits, relatively few published studies have explored the impact of a timely diagnosis of AD [42]. In one health-economic study, large clinical benefits occurred when patients received symptomatic AD medications eight years prior to standard diagnosis and Disease Modifying Treatments (DMT) two years prior to a standard diagnosis [44]. Other economic analyses reported that early identification and treatment of AD result in significant, positive social benefits and savings [45-47].
The current model, like other models of dementia patients, is comprehensive in that it includes repeat testing for patients who have received a misdiagnosis [24,30]. Researchers who did not include repeating testing recognize that their results cannot be extrapolated to the wider community or to repeated rounds of testing [27,48]. The current model is conservative, as it allows patients with a FN diagnosis to offset some of the extra progression-related costs once the patients are correctly diagnosed with subsequent testing.
The model also employs a conservative approach by including substantial CDP testing in combination with DISCERNTM. In a recent clinical utility study of 402 physicians, many providers indicated that they would use DISCERNTM in addition to the CDP until they were comfortable with the test, at which point they would reduce reliance on advanced imaging and biomarker testing [8]. Therefore, the opportunity exists for additional future savings with broader adoption of DISCERNTM. A scenario analysis in which DISCERNTM reduces the use of CT to 16%, MRI to 27% and CSF, PET, EEG and PSG to 0% results in overall threeyear net savings of $14.5 M for a Medicare Advantage plan with 1 M beneficiaries.
In 2021, Aduhelm (aducanumab), an amyloid-directed DMT, was granted FDA approval for the treatment of mild AD; however, the high cost of aducanumab and future DMTs will likely result in restrictions in their use (e.g., requiring a definitive diagnosis of AD) [8]. For example, the 2022 national coverage determination issued by the Centers for Medicare and Medicaid Services (CMS) states that CMS will cover new AD treatments involving amyloid beta-directed monoclonal antibodies if they either A) Receive FDA traditional (non-accelerated) approval or B) Are used with evidence of amyloid pathology consistent with AD in FDA- or NIH-approved trials for patients with a clinical diagnosis of mild cognitive impairment due to AD or mild AD dementia [10]. Thus, AD diagnostic tests with very high accuracy will become increasingly critical for drug development and disease management [42].
With the ability to assess critical AD factors beyond the levels of amyloid plaques alone, DISCERNTM provides clinical and economic impacts that will become more pronounced as more DMTs become available. Additionally, DISCERNTM can provide an opportunity for Medicare Advantage plans to improve star ratings through improved management of AD [49].
Limitations
This study is the first to estimate the economic impact associated with the use of DISCERNTM for AD; however, the study has limitations. First, for simplicity, the same performance characteristics were used for initial tests and testing in subsequent years and the sensitivity and specificity of the CDP are variable. We used estimates based on published data and analyzed the impact of performance characteristics in the sensitivity analysis.
Second, total costs of AD progression for patients without medication are unknown [50] but were estimated for the purposes of this model. These costs were then wholly or partially adjusted based on the patients’ stage of disease to account for the ability of AD medications to delay disease progression. Other models have used a single cost of AD progression that does not account for disease stage [30]. An additional scenario analysis using a single cost of AD progression increased savings to $11.6 M over three years for a Medicare Advantage plan with 1 M members.
Lastly, the model does not include indirect costs, such as quality of life outcomes for patients and caregivers, the impact on productivity, the direct costs of custodial care (such as adult day care) and home safety modifications; consequently, the model may not represent the full savings potential of DISCERNTM.
Conclusion
The economic burden of AD in the United States is substantial and projected to increase significantly by 2050, 5 signaling the clear need for early patient diagnosis and intervention to delay disease progression and improve patient outcomes. DISCERNTM shows promise as an accurate and definitive diagnostic tool for AD that results in savings for Medicare Advantage.
Funding
This work was supported with the financial assistance of NeuroDiagnostics, Inc. Rockville, MD, USA.
References
- Cardoso JR, Pereira LM, Iversen MD, Ramos AL (2014) What is gold standard and what is ground truth? Dental Press J Orthod 19: 27-30.
[Crossref],[Google scholar], [Indexed]
- American Academy of Neurology (2022) Practice guideline update summary for patients: Mild cognitive impairment.
- APA work group on Alzheimer's disease and other dementias, Rabins PV, Blacker D, Rovner BW, Rummans T, et al. (2007)American psychiatric association practice guideline for the treatment of patients with Alzheimer's disease and other dementias. Am J Psychiatry 164 : 5-56.
[Google scholar], [Indexed]
- Drabo EF, Barthold D, Joyce G, Ferido P, Chui HC, et al. (2019) Longitudinal analysis of dementia diagnosis and specialty care among racially diverse Medicare beneficiaries. Alzheimers Dement 15 :1402-1411.
[Crossref], [Google scholar], [Indexed]
- Alzheimer's association (2022) Alzheimer’s disease facts and figures.
- Moafmashhadi P, Koski L (2013) Limitations for interpreting failure on individual subtests of the Montreal cognitive assessment. J Geriatr Psychiatry Neurol 26 :19-28.
[Crossref], [Google scholar], [Indexed]
- Visser LNC, Pelt SAR, Kunneman M, Bouwman FH, Claus JJ, et al. (2020) Communicating uncertainties when disclosing diagnostic test results for (Alzheimer's) dementia in the memory clinic: The ABIDE project. Health Expect 23: 52-62.
[Crossref], [Google scholar], [Indexed]
- Samson C, Mark N, Datar M, Howell S, Huie F, et al. (2022) Physicians' assessment of the clinical utility of a novel test to diagnose Alzheimer's Disease (AD). Value in Health 27: 1-5.
- Beach TG, Monsell SE, Phillips LE, Kukull W (2012)Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005-2010. J Neuropathol Exp Neurol 71 :266-73.
[Crossref], [Google scholar], [Indexed]
- Centers for Medicare&Medicaid Services (2022) Monoclonal antibodies directed against amyloid for the treatment of Alzheimer’s Disease (CAG-00460N)-Decision memo.
- eviCore healthcare (2022) Clinical Guidelies: Head Imaging Policy.
- Khan TK, Alkon DL (2010) Early diagnostic accuracy and pathophysiologic relevance of an autopsy-confirmed Alzheimer's disease peripheral biomarker. Neurobiol Aging 31 :889-900.
[Crossref], [Google scholar], [Indexed]
- hirila FV, Khan TK, Alkon DL (2013) Spatiotemporal complexity of fibroblast networks screens for Alzheimer's disease. J Alzheimers Dis 33: 165-76.
[Crossref], [Google scholar], [Indexed]
- Khan TK, Sen A, Hongpaisan J, Lim CS, Nelson TJ, et al. (2015) PKCepsilon deficits in Alzheimer's disease brains and skin fibroblasts. J Alzheimers Dis 43: 491-509.
[Crossref], [Google scholar], [Indexed]
- Khan TK, Alkon DL (2006)An internally controlled peripheral biomarker for Alzheimer's disease: Erk1 and Erk2 responses to the inflammatory signal bradykinin. Proc Natl Acad Sci USA 103: 13203-13207.
[Crossref], [Google scholar], [Indexed]
- Sullivan SD, Mauskopf JA, Augustovski F, Caro JJ, Lee KM, et al. (2014) Budget impact analysis-principles of good practice: Report of the ISPOR 2012 budget impact analysis good practice II task force. Value Health 17: 5-14.
[Crossref], [Google scholar], [Indexed]
- Wilkinson D, Schindler R, Schwam E, Waldemar G, Jones RW, et al. (2009) Effectiveness of donepezil in reducing clinical worsening in patients with mild-to-moderate Alzheimer's disease. Dement Geriatr Cogn Disord 28: 244-251.
[Crossref], [Google scholar], [Indexed]
- Black SE, Doody R, Li H, Jambor KM, Xu Y, et al. (2007) Donepezil preserves cognition and global function in patients with severe Alzheimer disease. Neurology 69: 459-469.
[Crossref], [Google scholar], [Indexed]
- Davis M, Connell TO, Johnson S, Cline S, Merikle E, et al. (2018) Estimating alzheimer's disease progression rates from normal cognition through mild cognitive impairment and stages of dementia. Curr Alzheimer Res 15: 777-788.
[Crossref], [Google scholar], [Indexed]
- Kaiser Family Foundation (2022) Profile of Medicare beneficiaries March 2016 by race and ethnicity: A Chartpack.
- McMahon PM, Araki SS, Sandberg EA, Neumann PJ, Gazelle GS, et al. (2003) Cost-effectiveness of PET in the diagnosis of Alzheimer disease. Radiology 228: 515-522.
[Crossref], [Google scholar], [Indexed]
- Synaps Dx (2022) Data on file. Cross correlation of the three assays in the DISCERNTM test.
- Synaps Dx (2022) Data on file. Analytical performance for morphometric imaging assay.
- Guo S, Getsios D, Hernandez L, Cho K, Lawler E, et al. (2012) Florbetaben PET in the early diagnosis of Alzheimer's disease: A discrete event simulation to explore its potential value and key data gaps. Int J Alzheimers Dis 2012: 548157.
[Crossref], [Google scholar], [Indexed]
- What is gold standard and what is ground truth?
- Mainar AS, Vergara J, Colombo TL, Febrer L, Gutiérrez JR (2006) Análisis comparativo de los patrones de persistencia de medicaciones antidemencia en una cohorte retrospectiva de pacientes con demencia de tipo Alzheimer tratados con donepecilo, rivastigmina, galantamina y memantina en España. Rev Neurol 43: 449-453.
[Google scholar], [Indexed]
- McMahon PM, Araki SS, Neumann PJ, Harris GJ, Gazelle GS (2000) Cost-effectiveness of functional imaging tests in the diagnosis of Alzheimer disease. Radiology 217: 58-68.
[Crossref], [Google scholar], [Indexed]
- Matchar DB, Kulasingam SL, McCrory DC, Patwardhan MB, Rutschmann OT, et al. (2001) “Use of positron emission tomography and other neuroimaging techniques in the diagnosis and management of Alzheimer's disease and dementia.” AHRQ Technology Assessments.
[Crossref] [Google scholar], [Indexed]
- Podhorna J, Winter N, Zoebelein H, Perkins T (2020) “Alzheimer's treatment: Real-world physician behavior across countries.” Adv Ther 37: 894-905.
[Crossref], [Google scholar], [Indexed]
- Silverman DH, Gambhir SS, Huang HWC, Schwimmer J, Kim S, et al. (2022) Evaluating early dementia with and without assessment of regional cerebral metabolism by PET: A comparison of predicted costs and benefits. J Nucl Med 43: 253-66.
[Google scholar], [Indexed]
- Neumann PJ, Hermann RC, Kuntz KM, Araki SS, Duff SB, et al. (1999) Cost-effectiveness of donepezil in the treatment of mild or moderate Alzheimer's disease. Neurology 52: 1138-45.
[Crossref], [Google scholar], [Indexed]
- Robinson RL, Rentz DM, Andrews JS, Zagar A, Kim Y, et al. (2020) Costs of early stage Alzheimer's disease in the United States: Cross-sectional analysis of a prospective cohort study (GERAS-US). J Alzheimers Dis 75: 437-450.
[Crossref], [Google scholar], [Indexed]
- US Bureau of Labor Statistics (2022)Consumer Price Index (CPI) for Medical Care.
- American Psychological Association Services (2019) Psychological and neuropsychological testing billing and coding guide.
- Centers for Medicare & Medicaid Services (2022) April 2022 Ambulatory Surgery Center payment rates addenda. cms.gov.
- Centers for Medicare & Medicaid Services (2022) April 2022 Hospital OPPS Addendum A and Addendum B Updates. cms.gov.
- Centers for Medicare & Medicaid Services (2018) Medicare National Healthcare Common Procedure Coding System (HCPCS) Aggregate Report. DEPARTMENT of Health&Human Services.
- Optum EncoderPro. (2022) CPT® code section.
- PriceRx. https://pricerx.medispan.com/Refresh/Login.aspx.
- Deb A, Thornton JD, Sambamoorthi U, Innes K (2007) Direct and indirect cost of managing Alzheimer's disease and related dementias in the United States. Expert Rev Pharmacoecon Outcomes Res 17: 189-202.
[Crossref], [Google scholar], [Indexed]
- Happich M, Kirson NY, Desai U, King S, Birnbaum HG, et al. (2016) Excess costs associated with possible misdiagnosis of Alzheimer's disease among patients with vascular dementia in a UK CPRD population. J Alzheimers Dis 53: 171-83.
[Crossref], [Google scholar], [Indexed]
- Dubois B, Padovani A, Scheltens P, Rossi A, Dell'Agnello G (2016)Timely diagnosis for Alzheimer's disease: A literature review on benefits and challenges. J Alzheimers Dis 49: 617-31.
[Crossref], [Google scholar], [Indexed]
- Rasmussen J, Langerman H (2019) Alzheimer's disease-why we need early diagnosis. Degener Neurol Neuromuscul Dis 9: 123-130.
[Crossref], [Google scholar], [Indexed]
- Barnett JH, Lewis L, Blackwell AD, Taylor M (2014) Early intervention in Alzheimer's disease: A health economic study of the effects of diagnostic timing. BMC Neurol 14 :101.
[Crossref], [Google scholar], [Indexed]
- Handels RL, Wolfs CA, Aalten P, Joore MA, Verhey FR, et al. (2014) Diagnosing Alzheimer's disease: A systematic review of economic evaluations. Alzheimers Dement 10: 225-37.
[Crossref], [Google scholar], [Indexed]
- Banerjee S, Wittenberg R (2009)Clinical and cost effectiveness of services for early diagnosis and intervention in dementia. Int J Geriatr Psychiatry 24: 748-54.
[Crossref], [Google scholar], [Indexed]
- Weimer DL, Sager MA (2009) Early identification and treatment of Alzheimer's disease: Social and fiscal outcomes. Alzheimers Dement 5: 215-26.
[Crossref], [Google scholar], [Indexed]
- Larson EB, Reifler BV, Sumi SM, Canfield CG, Chinn NM (1985) Diagnostic evaluation of 200 elderly outpatients with suspected dementia. J Gerontol 40: 536-43.
[Crossref], [Google scholar], [Indexed]
- Better medicare alliance.Improving Medicare Advantage quality measurement.
- Green C, Handels R, Gustavsson A, Wimo A, Winblad B, et al. (2019) Assessing cost-effectiveness of early intervention in Alzheimer's disease: An open-source modeling framework. Alzheimers Dement 15: 1309-1321.
[Crossref], [Google scholar], [Indexed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences