An Updated Review on the Therapeutic Agents for Coronavirus Disease 2019
Muhammad Siraj, Maham Khan, Sadia Khan
Muhammad Siraj*, Maham Khan and Sadia Khan
Department of Biotechnology, University of Malakand, Chakdara, Pakistan
- Corresponding Author:
- Muhammad Siraj
Department of Biotechnology,
University of Malakand, Chakdara,
Pakistan
E-mail: sirajuom2@gmail.com
Received date: August 02, 2020; Accepted date: September 16, 2020; Published date: September 22, 2020
Citation: Siraj M, Khan M, Khan S (2020) An Updated Review on the Therapeutic Agents for Coronavirus Disease 2019. J Prev Med Vol. 5 Iss No. 4: 16.
Abstract
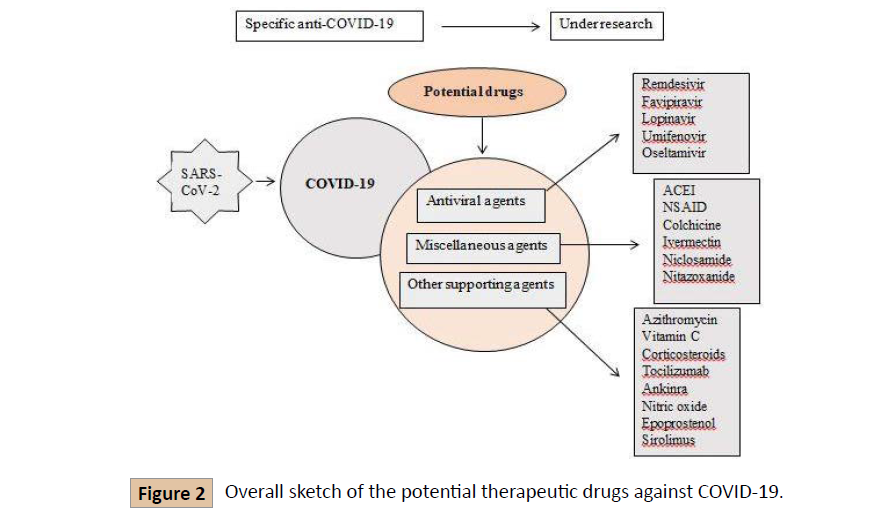
Coronavirus disease 2019 (COVID-19) emerged at the end of 2019 in Wuhan, China, and spread rapidly over other regions of the world. The pandemic coronavirus disease 19 (COVID-19) was caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Currently, there is no approved vaccine along with therapeutic agents available for the treatment of COVID-19. This review mainly focused on the most common and local drugs for the treatment of COVID-19. These therapeutic drugs include antiviral agents (Remdesivir, Lopinavir/Ritonavir, Umifenovir, Favipiravir, Oseltamivir), miscellaneous agents and therapies (Angiotensin-Converting Enzyme Inhibitors (ACEI), Non-steroidal anti-inflammatory drugs (NSAID), Colchicine, Convalescent Plasma therapy (CP therapy), Nitazoxanide), and other agents (Azithromycin, Vitamin C (Ascorbic acid), Corticosteroids and Dexamethasone), and Traditional Chinese Medicines. The material of this review is gathered from different research papers published on the aforementioned drugs for their potential use against COVID-19. However, none have enough evidence and requires more clinical trials.
Keywords
SARS-CoV-2, COVID-19, Treatment, Antiviral agents, Angiotensin- Converting Enzyme Inhibitors, Non-steroidal anti-inflammatory drugs, Convalescent plasma therapy
Introduction
Coronavirus disease 2019 (COVID-19) emerged at the end of 2019 in Wuhan, China. Its emergence is linked with Huanan Seafood Whole Sale Market where live animals were also sold [1]. Since then it has rapidly spread over other regions of the world and affected 209 countries. World Health Organization (WHO) revealed this outbreak as a pandemic on March 12, 2020 [2]. The novel Beta coronavirus is similar in genetics with severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) therefore it is officially named as SARS-CoV-2. It has expectedly emerged from bat originated coronavirus which is transmitted to humans through an intermediate mammal [3].
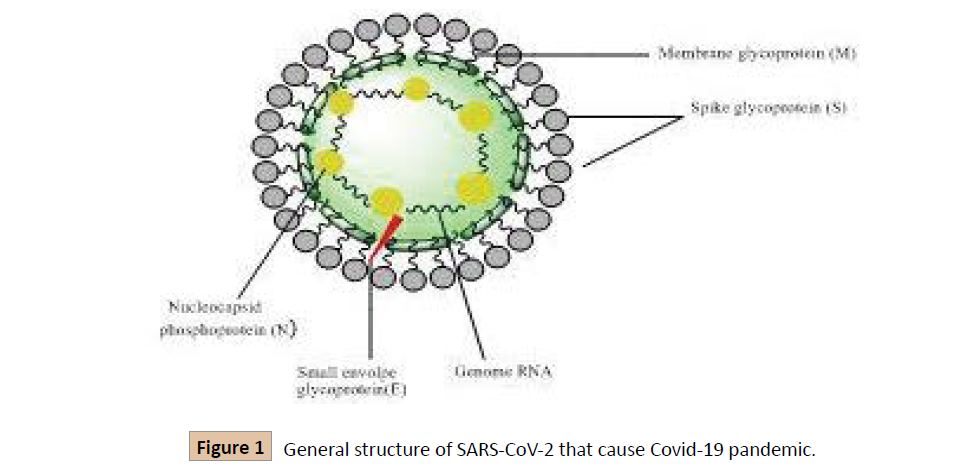
The Coronavirus family is surrounded by a protein envelope, having positive-sense single-stranded RNA that is about 26- 32kb in size [4]. Its genome is the biggest genomes amongst all recognized RNA viruses [5]. When SARS-CoV-2 enters the host cell using the ACE-2 (angiotensin-converting enzyme-2) receptor displayed by epithelial cells through spikes, its 5' open reading frame (ORF) is translated into long polypeptides which are sliced by protease enzyme encoded by the virus, and numerous nonstructural proteins are discharged. These proteins function to replicate the viral genome and also help to create nested copies that further helps in making viral proteins [6].
SARS-Cov-2 chiefly produces respiratory tract infections and certain strains have extreme infectivity and mortality [4]. The symptoms of COVID-19 appear after 5 days of incubation. The common symptoms include fever, cough, and fatigue, besides this sputum production, headache, shortness of breath, pneumonia, diarrhea, hemoptysis, and lymphopenia are also included [7]. The ease of the virus transmission from person to person and asymptomatic carriers has led to the rapid spread of the disease. The main routes for the transmission of coronavirus are droplets and close contact transmission. If a person coughs, sneezes breathe forcefully, or speak vocally the virus excretes from the body and potentially affects others nearby [8].
Millions of COVID-19 cases worldwide have been reported so far associated with a large proportion of deaths. The numbers of these cases are increasing day by day which held the healthcare system around the world on storm [9]. Since there are no approved vaccines to prevent the disease but are under clinical trials, certain preventive measures are adopted to reduce the spread of the disease. These measures include isolation of patients, adoption of appropriate Personal Protective Equipment (PPE) by members while providing clinical care to the patients such as wearing gloves, facial masks, gowns, and others, frequently washing hands mainly after contact with infected patients and also after sneezing and coughing [10]. Along with adopting preventive measures doctors and researchers throughout the globe are in a race to introduce effective medication to treat critically ill patients and reduce the death ratio of the affected population. For this purpose, many drugs are tested to determine their efficacy against SARS-CoV-2.
As so far there are no approved vaccines to prevent the disease, the world’s healthcare systems are also lacking approved therapeutic drugs to cure the disease and to date, they are under clinical trials. However, the medicinal drugs that were previously approved for other diseases such as hepatitis, HIV, etc. are proved to be advantageous in the treatment of COVID-19, as many diseases share overlying molecular paths their medications can be effectively reused until a cure is found for a new disease [11]. Antiviral, anti-inflammatory, convalescent plasma, herbal medicines, and RNA synthesis inhibitors are so far used to treat the COVID-19 patients and are discovered to be primary effective [8]. This review aims to transcribe the potential use of various drug therapies, for which several papers are reviewed to describe the conducted trials, mechanism of action, efficacy, and safety of various therapeutic drugs.
Antiviral Agents Based Strategies
Remdesivir
Remdesivir (GS-5734) is a pro-drug analog of adenosine nucleotide which shows broad-spectrum antiviral activity against different RNA viruses. Currently, it is under clinical development for the treatment of Ebola virus disease (EVD). In cell culture, their antiviral effects have been demonstrated and also in nonhuman primates [12]. It is an experimental drug and synthesized by Gilead Sciences in 2017. The in vitro studies showed that remdesivir can be used for the treatment of coronavirus that inhibits SARS-CoV and MERS-CoV replication. Remdesivir is metabolized in its active form, which joints to the nascent RNA resulting in pre-mature termination, preventing viral replication [13].
In China on January 31, 2020, there are two phases 3 clinical trials that are randomized, multicenter, placebo-controlled; double-blind to evaluate the safety and efficacy of the drug in the patient of COVID19. Through intravenous infusion, the initial dose of remdesivir is 200 mg received by the experimental group followed by 100 mg for 9 consecutive days, in addition to routine treatment. In the control group, patients received the same dose of placebo treatment. In April 2020, these trails were ended [14,15]. NIH press released, that described the moderate benefit of remdesivir, and the experts are encouraged by their benefit but it is still difficult to know about the best use of remdesivir. Without confirm data, it is hoped that remdesivir reduces the patient viral burden. During any emergency, the FDA allowed the use of remdesivir outside of clinical trials [16].
Lopinavir/Ritonavir (LPVr)
These are the antiretroviral medications, marketed and administered exclusively in combination. This combination was marketed for the first time by Abbot in 2000 under the marketed name Kaletra. Lopinavir is co-formulated with ritonavir because of lopinavir has poor biotransformation as well as poor oral bioavailability. Their co-administration boosts lopinavir exposure and improves their antiviral activity. Lopinavir is a protease inhibitor that is widely used for HIV treatment [9]. Currently, for the treatment of coronavirus disease, there is no perfect evidence for the efficacy of lopinavir/ritonavir (LPVr). There are several clinical trials of lopinavir/ritonavir (LPVr) that are currently ongoing against COVID19. Non-randomized, openlabel in vivo study was found that reduced the rate of deaths in SARS-CoV patients, who were treated with LPVr antiviral drugs. LPVr is a protease inhibitor that inhibits the action of enzyme 3-chymotrypsin-like protease (3CLpro), that disrupt the viral replication process and release from the host cell [17].
Umifenovir (branded as Arbidol)
For the first time, the Umifenovir was developed in Russia in 1988. It is approved by Russia and China for the treatment of influenza, prophylaxis, and other arboviruses. Later on, in vitro study was demonstrated that it has antiviral efficacy against the Ebola virus, human herpesvirus 8 (HHV8), hepatitis C and many more [9]. The retrospective study has reported that Umifenovir treatment did not increase the negative conversion rate of SARSCoV patients. Nowadays there are no vaccines available for COVID19 treatment but it is an urgent effective broad-spectrum antiviral medication for the treatment of COVD19. An in vitro study has reported that Umifenovir inhibits SARS-CoV replication in GMK-AH-1 cells. It was recommended by china for prevention and guidelines [18].
Favipiravir (branded as Avigan)
Favipiravir is an antiviral medication that inhibits RNA-dependent RNA polymerase (RdRp) of RNA viruses selectively and potently. The efficacy of the replication of viral RNA reduces by competitive inhibitor. It was discovered by Toyama Chemical Co., Ltd against influenza viruses in Japan in 2014. Instead of an influenza virus, Favipiravir also shows antiviral activities against other RNA viruses such as arenaviruses, bunyaviruses, and many more [19,20]. In vitro studies of Favipiravir showed that it has the activity against SARS-CoV-2 but it has required higher concentration as compared to other antiviral medications. A non- randomized and open-label trial was done in China on 80 COVID19 patients, a clear reduction (recovery) have occurred in patients treated with Favipiravir [21].
Oseltamivir (branded as Tamiflu)
Oseltamivir is a pro-drug inhibitor that inhibits the neuraminidase glycoprotein selectively and potently which is important for the replication of influenza A and B viruses [22]. Oseltamivir was reported in China during the COVID19 epidemic outbreak, used either with or without corticosteroids or antibiotics. It is also used in clinical trials with association with Favipiravir and other antiviral medications. In Wuhan, a study reported that no benefit was observed when COVID19 patients were treated with Oseltamivir. For SARS-CoV-2 disease, several clinical trials still ongoing to improve the effectiveness of Oseltamivir [9,23].
Miscellaneous Agents Based Strategies
Angiotensin-Converting Enzyme Inhibitors (ACEI)
COVID-19 patients with hypertension and coronary heart disease form a peril ratio of deaths and acute respiratory death syndrome [24,25]. The principal line of medication for hypertension is the use of ACEIs (angiotensin-converting enzyme inhibitors) and ARBs (angiotensin-2 receptor blockers) [26]. Various studies have revealed that the use of ACEI/ARB increases the expression of ACE-2 (angiotensin-converting enzyme-2) receptors by epithelial cells of the lung, intestine, kidney, and blood vessels, which provide the site of entry for SARS-Cov-2 [27]. The mechanism for SARS-CoV-2 infection involves the condition of availability of the receptor on the surface of the cells, and ACE-2 is recognized as a co-receptor for the binding and entry of the virus, where the virus binds through its spike protein and enters the cell through endocytosis [28]. ACE-2 is a type 1 transmembrane protein, its enzymatic domain occurs at the outer surface of the cell where it plays the activity of converting angiotensin-2 (1-8) to 1-7 known as a vasodilator. However, the entrance of the virus to the cell results in the decrease of ACE-2 expression which might be the response of innate immunity [29]. It has been hypothesized by some studies that the use of ACEI/ARB for patients with hypertension and cardiovascular diseases increases the chance of occurring lethal COVID-19 as it increases the expression of the ACE-2 receptor [30].
To determine the effect of the use of antihypertensive drugs on COVID-19 patients, researches have been conducted between the patients receiving ACEI/ARB and patients not receiving ACEI/ARB and no significant differences have been observed. However, the use of antihypertensive drugs is proved to be positively effective [31]. To validate the use of ACEI for COVID-19 patients with cardiovascular diseases requires more clinical trials. Sacubitril/valsartan is revealed to be an effective therapeutic for cardiovascular diseases as it is reported to stabilize heart failure and showed to be effective in improving the health of COVID-19 patients with heart diseases [32]. Employing sartans for COVID-19 patients are also considered to protect them from potential acute lung injury. Neprilysin with its inhibitor Sacubitril reduces the neutrophil and pro-inflammatory cytokine count and increases lymphocyte count (which is found to be the indicator of COVID-19 surviving patients) in patients with acute heart failure [33]. It is suggested that the use of ACEI/ARB is advantageous because it inhibits the level of angiotensin-2 which is known to increase during SARS-CoV-2 infection. Thus it is safe to use, conversely, there is no clinical confirmation and hence requires further clinical trials [34].
Non-steroidal anti-inflammatory drugs (NSAID)
NSAIDs block the production of prostaglandins which are the main intermediaries of fever and inflammation, by inhibiting cyclooxygenase 1 and 2 [35]. These drugs are largely used for the treatment of arthritis and can potentially prevent the lethal cytokine storm of COVID-19 [36]. The NSAID of concern with COVID-19 is Ibuprofen and Indomethacin. Ibuprofen is a commonly recommended drug and is found to decrease IL-6 in human tissues 2. The use of it was considered to worsen the condition of COVID-19 patients as it can potentially overexpress the ACE-2 receptors but now it is declared by the European Medicine Agency that the link between deteriorating COVID-19 and ibuprofen does not hold any scientific evidence however precautions must be taken and alternative drugs such as aspirin and paracetamol to treat pain and pyrexia should be used until a piece of scientific evidence is obtained [37].
Indomethacin trial in 2006 has been conducted in dogs and is found to be an effective antiviral treatment against Canine Coronavirus as it inhibits the viral replication, similar activity is also found in humans against SARS-CoV [38]. This suggests the probable efficacy against SARS-CoV-2.
Colchicine
Colchicine is a lipid-soluble alkaloid that accrues in granulocytes and monocytes with resultant anti-inflammatory influence; it is also documented to be inhibiting inflammasomes which are considered as a main pathophysiological component in the medical course of COVID-19 patients and moderating interleukin activation [39]. Instantly colchicine is proved to be a favorable drug to regulate natural immunity dysregulation in COVID-19 [40]. Trials are conducted on the use of colchicine and are proved to be effective in reducing cytokine storms. The outcome of the GRECCO-19 trial advises that colchicine is safe and may recover outcomes in COVID-19 patients. However, this requires a sizeable study and therefore 12 trials are on their way to determine the efficacy of colchicines [41].
Ivermectin and Niclosamide
Ivermectin is a satisfactory anti-helminthic drug that is well known for its ability to paralyze the infesting organism by hyperpolarizing through the stimulation of gamma-aminobutyric acid gated chloride channels and is also known to boost host immunity [42]. It is found to be effective against various viruses such as HIV, dengue, West Nile Virus, and influenza. Freshly, it is found to be effective against COVI-19 in in-vitro cell cultures [43]. Lately, Caly et al established antiviral activity of ivermectin after 24-48 hours of a single dose in in-vitro cultures however in vivo studies have not been conducted [44]. The normal dose of this drug is reflected to be safe in human therapy [45].
Niclosamide is an anthelmintic drug approved by the FDA that has been broadly used for treating tapeworm infection and is recorded as one of the essential medicines by the World Health Organization. It can stimulate several signaling pathways and treat various human diseases like cancer, viral and bacterial infections, and metabolic diseases [46]. The in vitro experiments showed its effectiveness in low concentrations for inhibiting replication of SARS-CoV, MERS-CoV, zika virus, hepatitis C virus, and human adenovirus [47].
Niclosamide presents very potent antiviral activity against SARSCoV- 2, as its ability to lower MERS-CoV replication by inhibiting SKP2 activity is demonstrated by Gassen et al. A pharmacokinetic defect of niclosamide i.e. low absorption requires further development or design of the drug for the safe and effective delivery [48].
Nitazoxanide
Nitazoxanide and its functional derivative nitazoxanide have proved effective in-vitro against SARS-CoV-2. It is believed to have a wide range of antiviral activity because it interferes with host regulated pathways implicated in viral replication preferably than virus particular pathways [49]. It is an antiprotozoal drug approved by the FDA to treat cryptosporidium and Giardia. It has brilliant safety evidence for diverse clues and is used in the treatment of several viruses including hepatitis B, C, Rotavirus, and Norovirus. Considering the efficacy and safety of this drug in the use of different viral diseases it should also be studied in trials for its effectiveness against COVID-19 [50].
Convalescent plasma therapy (CP therapy)
The practice of convalescent plasma or immunoglobins was suggested as an experiential treatment during the Ebola virus pandemic in 2014 and MERS-CoV in 2015. The same method was also proved effective in the treatment of other viral diseases such as SARS-CoV, avian influenza, and H1N1 influenza [51]. The randomized clinical trials on the use of convalescent plasma for the treatment of severe influenza A were linked with a decreased viral load and lower mortality, same was the case with SARSCoV [52]. CP therapy could be avowing treatment for COVID-19 patients. Patients cured of the disease with high counteracting antibody titer may be a worthy donor basis of CP. Studies conducted on the treatment of COVID-19 patient with CP proves to be very effective as it results in decreased mortality and a shorter stay in hospital [53].
The antibodies of convalescent plasma can facilitate their therapeutic effect through diverse mechanisms i.e. the antibodies can directly neutralize the antigen by adhering to it, though other pathways of humoral immunity such as complement system, phagocytosis, and antibody-mediated cellular cytotoxicity can also influence its therapeutic effect. Currently, the only available effective treatment for COVID-19 is the use of anti-SARS-Cov-2 plasma therapy [54]. The US Food and Drug Administration has approved the use of plasma from recovered patients to treat seriously ill patients [55].
Other Therapeutic Agents
Azithromycin
Azithromycin is a macrolide antibiotic used for acne vulgaris and skin, respiratory and other infections. It has shown in vivo activity in the prevention of severe respiratory tract involvement in viral infections and in vitro activity against Ebola and Zika viruses [56]. It prevents the growth of bacteria by interfering with their protein synthesis. It stops the translation of mRNA, by the attachment with the 50S subunit of the bacterial ribosome [57]. Clinical trials are conducted by UC San Francisco which is hiring more than 2,500 adults who are newly spotted with COVID-19 to assess whether the common antibiotic azithromycin can reduce hospitalization stays and deaths caused by Covid-19. Molina et al assessed clinical and virologic outcomes of 11 consecutive hospitalized patients who received azithromycin (500mg on day 1, then 250 mg days 2-5) and hydroxychloroquine (600 mg/day for 10 days). In this study 4 women and 7 men were included; 8 had significant comorbidities related to poor outcomes. Among 11, 10 patients had a fever and relied on ventilators. Within 5 days, two patients were transferred to the ICU and one patient died. The conducted study shows no evidence of a clinical benefit or antiviral activity conferred by hydroxychloroquine plus azithromycin (Figures 1 and 2) [58].
Vitamin C (Ascorbic acid)
Vitamin C is a super nutrient which our body cannot synthesize and we need to get it from exogenous sources [59]. Vitamin C plays its role in the maintenance of the immune system through its antioxidant ability, collagen synthesis or directly strengthening immune cells in the fight against infection [60]. In China and Italy, a high dose of vitamin C IV is used to cure the lung functions of Covid-19 patients. A solution of vitamin C is delivered directly into the bloodstream, in the blood, its concentration raises very quickly. However, Vitamin C's efficacy is still being tested [61].
Corticosteroids and dexamethasone
Corticosteroids have a good inhibitory effect on inflammatory factors and are often used as a supporting treatment for viral pneumonia. The main anti-inflammatory effect of glucocorticoids is to obstruct a large number of pro-inflammatory genes that encode chemokines, cytokines, and cell adhesion molecules, receptors to address the inflammatory process, inflammatory enzymes and restore homeostasis [62]. Dexamethasone is used since the 1960s as a steroid to reduce inflammation. Pakistan will continue using dexamethasone to treat critical novel coronavirus patients. This is the first treatment to be shown to reduce mortality in patients with COVID-19 requiring oxygen on ventilator support, WHO addressed it as a lifesaving breakthrough [63]. The WHO conducted clinical trials that show dexamethasone, corticosteroids, can save the life of COVID-19 patients. For patients on ventilators, the treatment was shown to reduce mortality about one-third, and those patients requiring only oxygen the mortality rate was cut about one-fifth. The benefit was observed only in those patients who are critically ill not seen in patients with milder symptoms [64].
Nitric oxide and Epoprostenol
Inhaled nitric oxide and epoprostenol have been used for pulmonary vasodilation for vasoreactivity testing, post-cardiac surgery patients, patients with acute respiratory distress syndrome (ARDS). In the COVID-19 era, there are concerns about the generation of aerosol when using a nebulizer for inhaled pulmonary vasodilators. Nitric oxide inhalation is currently investigated as a preventive measure and treatment against COVID-19 (e.g. clinical trials NCT04306393, NCT04312243, NCT04338828, and NCT04305457) [64]. Inhalation of NO is a treatment to improve arterial oxygenation against acute respiratory distress syndrome, which signifies a major problem of COVID-19. On March 20, 2020, FDA allows expanding the emergency use of inhaled NO (INO pulse) to be used immediately for the treatment of COVID-19 [57].
Sirolimus
Covid-19 is globally a major threat; an additional drug is required to boost immune response in a vaccinated but weakened person. Sirolimus is known as rapamycin, it is used as an immunosuppressant to prevent rejection of organ transplant and to treat lymphangioleiomyomatosis by inhibiting the mammalian target of rapamycin (mTOR) kinase. mTOR, mTORC1 is a protein complex formed by mTOR that plays its role in viral replication. In an in-vitro experiment, sirolimus is shown to affect the PI3K/ AKT/mTOR pathway which inhibited MERS-CoV activity and has the potential to treat COVID 19 [65].
Tocilizumab
In COVID-19 patients, a large number of mononuclear macrophages and T lymphocytes are activated, producing cytokines such as interleukin-6, which bind to the IL-6 receptor on the target cell, causing the cytokine response and severe inflammatory responses in lungs and other tissues and organs. Tocilizumab, is an anti-human IL-6 receptor monoclonal antibody, can bind to the IL-6 receptor thus preventing IL-6 binding to its receptor, preventing the immune damage to the target cells, and alleviating the inflammatory responses [66]. 21 patients from the first affiliated Hospital of China were diagnosed as severe or critical COVID-19 patients and were given tocilizumab therapy. The results were very interesting, the temperature of all the patients reduced quickly along with the improvement of other symptoms. Among the 21 patients, 20 recovered and discharged within 2 weeks. One left patient recovered out of ICU care. No side effects of the drug are reported during this therapy [67].
Anakinra
Anakinra is a 17 KD recombinant, non-glycosylated human IL-1 receptor competitor with a short half-life of about 3-4 h and a good safety profile. SARS-CoV-2 activates the inflammasome by binding to toll-like receptors and the cleavage of pro-IL-1 by caspase-1, followed by the production of mature IL-1, an intermediary of fever, fibrosis, and lung inflammation. Coronavirus encodes viroporins, open reading frame 3a, and viroporins E can induce transcription of the gene encoding pro-IL-1 and secrete IL-1 by the activation of the NLRP3 inflammasome. Anakinra drug is involved in the inflammasome pathway which represents an efficient treatment of Covid-19 [68].
Traditional Chinese Medicines (TCM) Based Strategies
WHO (World Health Organization) concluded from the previous persistent analysis that ''to date, there are no specific medications available for the prevention or treatment of SARS- CoV-2'' in China. Historically, in China when the outbreak of SARS-CoV-2 occurred, oral administration of Chinese medicine (CM) was recommended for prevention and treatment against SARSCoV- 2. These include preventive herbal formulae, indoor herbal medicine fumigation, etc. It was reported that COVID19 patients have benefited from the TCM treatment. It showed the positive outcomes that decrease death rates and reliefs from symptoms [34,63]. It was reported that more than 85% of patients in China had taken TCM for treatment. SARS-CoV-2 has similar to SARSCoV which uses host receptor ACE2 for cell entrance, some studies showed that the TCM may have targeted this receptor and prevent the disease of SARS-CoV-2. Due to the similarity between SARS-CoV and SARS-CoV-2, some herbal medicines were used for the treatment of SARS-CoV-2 in Korea and China (Tables 1-3).
| Group | Drugs | Mechanism of action | Original use | Ref* |
|---|---|---|---|---|
| RNA synthesis inhibitors | Remdesivir | Inhibit the action of RdRp of RNA viruses | Ebola Haemorrhagic Virus (EBV) and Marburg infection treatment | [24] |
| RNA synthesis inhibitors | Favipiravir | Inhibit the action RdRp of RNA viruses | Influenza and other virus treatment | [19,20] |
| Viral protein synthesis inhibitors | Lopinavir/ritonavir | Inhibit the action of 3CLpro | HIV treatment | [17] |
| Viral membrane inhibitor | Umifenovir | Block the virus-cell membrane and endosome fusion | Influenza and other virus treatment | [9] |
| Neuraminidase inhibitor | Oseltamivir | Inhibit the neuraminidase glycoprotein | Influenza A and B virus treatment | [22] |
Table 1: Table showing antiviral agents, their mechanism of action, and original uses.
| Group | Drugs | Mechanism of action | Original use | Ref* |
|---|---|---|---|---|
| ACE inhibitors | Captopril, enalapril, fosinopril, sacubitril | Inhibits the formation of angiotensin-2, pro-inflammatory cytokines | Hypertension, cardiovascular diseases | [25] |
| NSAIDs | Ibuprofen and indomethacin | Non selectively inhibits cyclooxygenase involved in prostaglandin synthesis | Mild to moderate pain, inflammation, fever. | [35] |
| Anti-inflammatory | Colchicine | Inhibits microtubular polymerization, IL-1 and 6, NLRP3 inflammasome | Gouty arthritis, familial Mediterranean fever (FMF) | [39] |
| Anti-parasitic | Ivermectin | Stimulate GABA-gated Cl channels, inhibit importin 1 heterodimer to inhibit host nuclear and viral proteins import | Parasitic infestation | [42] |
| Niclosamide | Stimulate several signaling pathways in humans, inhibits glucose uptake, oxidative phosphorylation, and anaerobic metabolism in tapeworm. | Tapeworm infection | [46] | |
| Immunomodulators | Nitazoxanide | Inhibit host controlled pathways implicated in viral replication, intensifying RNA sensing and type 1IFN pathways | Cryptosporidium and Giardia | [49] |
Table 2: Table showing miscellaneous agents, their mechanism of action, and original uses.
| Group | Drugs | Mechanism of action | Original use | Ref* |
|---|---|---|---|---|
| Antibiotics | Azithromycin | It stops the translation of mRNA, by the attachment with the 50S subunit of the bacterial ribosome. | Skin, respiratory, urogenital, and other infections. | [60] |
| Anti-inflammatory | Corticosteroids | Inhibit the release of phospholipase A2, inhibit inflammatory transcription factors, increase expression of anti-inflammatory genes | Rheumatoid arthritis, psoriasis. | [69] |
| Immunosuppressant | Sirolimus | Inhibit the mammalian target of rapamycin complex 1, block vial protein expression | Prevent organ transplant rejection | [65] |
| Monoclonal antibody | Tocilizumab | Inhibits the binding of IL-6 to its receptor. | Rheumatoid Arthritis and juvenile idiopathic arthritis (JIA). | [70] |
| Inter leukin-1 receptor competitor | Anakinra | Involved in the Inflammasome pathway. | Hyper inflammation | [68] |
Table 3: Table showing other Supporting agents, their mechanism of action, and original uses.
In China for the COVID-19 patients, the top 10 TCM herbal medicines were commonly used include; Astragalus membranaceus, Saposhnikoviae divaricate, Rhizoma Atractylodis Macrocephalae, Glycyrrhiza uralensis, Lonicerae Japonicae Flos, Fructus forsythia, Atractylodis Rhizoma, Radix platycodonis, Agastache rugosa, and Cyrtomium fortunei J. Sm [69]. Traditional herbal medicine is a mixture of herbs prescribed by Chinese herbalists. During the outbreak of such an epidemic of SARSCoV- 2, Chinese herbal medicine has been reported as successfully prevented and treated drugs against SARS-CoV-2. Furthermore, to reduce the adverse effect the Chinese herbal medicine was combined with western medicine induced by corticosteroids, antiviral and antibiotic treatments [70].
Vaccines for COVID-19
While this situation researchers around the globe are focusing on vaccine development against SARS-CoV-2 to protect against COVID-19. Worldwide current efforts are to find an effective vaccine against the SARS-CoV-2. The expectation is that under the use of emergency protocol the first licensed vaccine may available in early 2021 [71,72].
Conclusion
This review is an attempt to shed light on all recently used therapeutic agents, and their efficacy level against novel SARSCoV- 2. Hopefully, the SARS-CoV-2 vaccine and other specific targeting drugs will be made and available in markets in the next few months or years. During the ongoing clinical research on COVID-19 to develop a potential drug for this disease, our hope will be on the horizon.
References
- Hui DS, Azhar EI, Madani TA, Ntoumi F, Kock R, et al. (2020) The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis 91:264-266.
- Waris A, Khan AU, Ali M, Ali A, Baset A (2020) COVID-19 outbreak: current scenario of Pakistan. New Microbes and New Infections 14:100681.
- Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB (2020) Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA 323:1824-1836.
- Waris A, Ali M, Khan AU, Ali A, Bangash AK, et al. (2020) COVID-19 Incidence in Pakistan: Gender Disparity. Iranian Journal of Psychiatry and Behavioral Sciences: (In Press) e105990.
- Woo PCY, Huang Y, Lau SKP, Yuen KY (2010) Coronavirus genomics, and bioinformatics analysis. Viruses 2:1804-1820.
- Marra MA, Jones SJM, Astell CR, Holt RA, Brooks-Wilson A, et al. (2003) The genome sequence of the SARS-associated coronavirus. Science 300:1399-1404.
- Rothan HA, Byrareddy SN (2020) The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun 109:102433.
- Wang J, Du G (2020) COVID-19 may transmit through aerosol. Ir J Med Sci 1-2.
- Wu R, Wang L, Dina Kuo H-C, Shannar A, Peter R, et al. (2020) An update on current therapeutic drugs treating COVID-19. Curr Pharmacol Rep 1-15.
- Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Napoli RD (2020) Features, evaluation, and treatment coronavirus (COVID-19), in Statpearls [internet]. StatPearls Publishing.
- Waris A, Ali M, Khan AU, Ali A, Baset A (2020) Role of nanotechnology in diagnosing and treating COVID-19 during the Pandemic.Int J Clin Virol 4:065-070.
- Tchesnokov EP, Feng JY, Porter DP, Gotte M (2019) Mechanism of inhibition of Ebola virus RNA-dependent RNA polymerase by remdesivir. Viruses 11:326.
- Al-Tawfiq JA, AH Al-Homoud, Memish ZA (2020) Remdesivir as a possible therapeutic option for the COVID-19. Travel Med Infect Dis 34:101615.
- Wang M, Cao R, Zhang L, Yang X, Liu J, et al. (2020) Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 30:269-271.
- Ko W-C, Rolain J-M, Lee N-Y, Chen P-L, Huang C-T, et al. (2020) Arguments in favor of remdesivir for treating SARS-CoV-2 infections. Int J Antimicrob Agents 55:105933.
- Nina B (2020) Antiviral Drug Remdesivir Can Help Fight the Coronavirus, But Can Patients Get It? University of California San Francisco (UCSF).
- Dorward J, Gbinigie K (2020) Lopinavir/ritonavir: A rapid review of effectiveness in COVID-19.
- Lian N, Xie H, Lin S, Huang J, Zhao J, et al. (2020) Umifenovir treatment is not associated with improved outcomes in patients with coronavirus disease 2019: a retrospective study. Clin Microbiol Infect 26:917–921.
- Furuta Y, Komeno T, Nakamura T (2017) Favipiravir (T-705), a broad-spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B Phys Biol Sci 93:449-463.
- Yanai H (2020) Favipiravir: A Possible Pharmaceutical Treatment for COVID-19. Journal of Endocrinology and Metabolism 10:33-34.
- Coomes EA, Haghbayan H (2020) Favipiravir, an antiviral for COVID-19? Journal of Antimicrobial Chemotherapy 75:2013-2014.
- McClellan K, Perry CM (2001) Oseltamivir. Drugs 61:263-283.
- Rosa SGV, Santos WC (2020) Clinical trials on drug repositioning for COVID-19 treatment. Rev Panam Salud Publica 44:e40.
- Saha A, Sharma AR, Bhattacharya M, Sharma G, Lee S-S, et al. (2020) Probable Molecular Mechanism of Remdesivir for the Treatment of COVID-19: Need to Know More. Arch Med Res 51:585-586.
- Patel AB, Verma A (2020) COVID-19 and angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: what is the evidence? JAMA 323:1769-1770.
- Zhang P, Zhu L, Cai J, Lei F, Qin J-J, et al. (2020) Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res 126:1671-1681.
- Guo J, Huang Z, Lin L, Jiagao LV (2020) Coronavirus disease 2019 (COVID‐19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infections. J Am Heart Assoc 9:e016219.
- South AM, Diz DI, Chappell MC (2020) COVID-19, ACE2, and cardiovascular consequences. Am J Physiol Heart Circ Physiol 318:H1084-H1090.
- Liu PP, Blet A, Smyth D, Li H (2020) The science underlying COVID-19: implications for the cardiovascular system. Circulation 142:68-78.
- Fang L, Karakiulakis G, Roth M (2020) Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med 8:e21.
- Liu Y, Huang F, Xu J, Yang P, Qin Y, et al. (2020) Anti-hypertensive Angiotensin II receptor blockers associated with mitigation of disease severity in elderly COVID-19 patients. MedRxiv.
- Acanfora D, Ciccone MM, Scicchitano P, Acanfora C, Casucci G (2020) Sacubitril/valsartan in COVID-19 patients: the need for trials Eur Heart J Cardiovasc Pharmacother 6:253-254.
- Acanfora D, Ciccone MM, Scicchitano P, Acanfora C, Casucci G (2020) Neprilysin inhibitor–angiotensin II receptor blocker combination (sacubitril/valsartan): rationale for adoption in SARS-CoV-2 patients. Eur Heart J Cardiovasc Pharmacother 6:135-136.
- Meng J, Xiao G, Zhang J, He X, Ou M, et al. (2020) Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect 9:757-760.
- Mehta N, Mazer-Amirshahi M, Alkindi N, Pourmand A (2020) Pharmacotherapy in COVID-19; A narrative review for emergency providers. Am J Emerg Med 38:1488–1493.
- Giollo A, Adami G, Gatti D, Idolazzi L, Rossini M (2020) Coronavirus disease 19 (Covid-19) and non-steroidal anti-inflammatory drugs (NSAID). Ann Rheum Dis.
- Day M (2020) Covid-19: European drugs agency to review the safety of ibuprofen. BMJ 368:m1168.
- Russell B, Moss C, Rigg A, Hemelrijck MV (2020) COVID-19 and treatment with NSAIDs and corticosteroids: should we be limiting their use in the clinical setting? Ecancermedicalscience 14:1023.
- Deftereos S, Giannopoulos G, Vrachatis DA, Siasos G, Giotaki SG, et al. (2020) Colchicine as a potent anti-inflammatory treatment in COVID-19: can we teach an old dog new tricks? Eur Heart J Cardiovasc Pharmacother pvaa033.
- Ferro F, Elefante E, Baldini C, Bartoloni C, Puxeddu I, et al. (2020) COVID-19: the new challenge for rheumatologists. Clin Exp Rheumatol 38:175-180.
- Rabbani AB, Parikh RV, Rafique AM (2020) Colchicine for the Treatment of Myocardial Injury in Patients With Coronavirus Disease 2019 (COVID-19)—An Old Drug With New Life? JAMA Netw Open 3:e2013556.
- Gupta D, Sahoo AK, Singh A (2020) Ivermectin: a potential candidate for the treatment of COVID 19. Braz J Infect Dis 24:369–371.
- Kumar R, Gupta N, Kodan P, Mittal A, Soneja M, et al. (2020) Battling COVID-19: using old weapons for a new enemy. Trop Dis Travel Med Vaccines 6:1-10.
- Patrì A, Fabbrocini G (2020) Hydroxychloroquine, and ivermectin: A synergistic combination for COVID-19 chemoprophylaxis and treatment? J Am Acad Dermatol 82:e221.
- Jean SS, Hsueh P-S (2020) Old and re-purposed drugs for the treatment of COVID-19. Expert Rev Anti Infect Ther 1-5.
- Xu J, Shi P-Y, Li H, Zhou J (2020) Broad-spectrum antiviral agent niclosamide and its therapeutic potential. ACS Infect Dis 6:909-915.
- Paumgartten FJR, Delgado IF, Pitta LDR, De-Oliveira ACAX (2020) Drug repurposing clinical trials in the search for life-saving Covid-19 therapies; research targets and methodological and ethical issues. Vigilância Sanitária em Debate 8:39-53.
- Jeon S, Ko M, Lee J, Choi I, Byun SY, et al. (2020) Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs. Antimicrobial Agents and Chemotherapy.
- Yavuz SS, Ünal S (2020) Antiviral treatment of COVID-19. Turk J Med Sci 50:611-619.
- Srivatsan P (2020) Potential dual therapeutic approach against SARS-CoV-2/COVID-19 with Nitazoxanide and Hydroxychloroquine.
- Shen C, Wang Z, Zhao F, Yang Y, Li J, et al., (2020) Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA 323:1582-1589.
- Chen L, Xiong J, Bao L, Shi Y (2020) Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis 20:398-400.
- Duan K, Liu B, Li C, Zhang H, Yu T, et al. (2020) Effectiveness of convalescent plasma therapy in severe COVID-19 patients. PNAS 117:9490-9496.
- Bloch EM, Shoham S, Casadevall A, Sachais BS, Shaz B, et al. (2020) Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest 130:2757-2765.
- Tanne JH (2020) Covid-19: FDA approves the use of convalescent plasma to treat critically ill patients. BMJ 368:m1256.
- Schwartz RA, Suskind RM (2020) Azithromycin, and COVID‐19Prompt Early Use at First Signs of this Infection in Adults and Children An Approach Worthy of Consideration. Dermatologic Therapy e13785.
- Wu R, Wang L, Kuo H-CD, Shannar A, Peter R, et al. (2020) An update on current therapeutic drugs treating COVID-19. Current Pharmacol Rep 1-15.
- Carlucci P, Ahuja T, Petrilli CM, Rajagopalan H, Jones S, et al. (2020) Hydroxychloroquine and azithromycin plus zinc vs hydroxychloroquine and azithromycin alone: outcomes in hospitalized COVID-19 patients. medRxiv.
- Mashour S, Turner Jr JF, Merrell R (2000) Acute renal failure, oxalosis, and vitamin C supplementation: a case report and review of the literature. Chest 118:561-563.
- Parnham MJ, Haber VE, Giamarellos-Bourboulis EJ, Perletti G, Verleden GM, et al. (2014) Azithromycin: mechanisms of action and their relevance for clinical applications. Pharmacol Ther 143:225-245.
- Carr AC, Maggini S (2017) Vitamin C and immune function. Nutrients 9:1211.
- Yang Z, Liu J, Zhou Y, Zhao X, Zhao Q, et al. (2020) The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis. J Infect 81:e13-e20.
- Luo H, Tang Q-L, Shang Y-x, Liang S-b, Yang M, et al. (2020) Can Chinese medicine be used for the prevention of coronavirus disease 2019 (COVID-19)? A review of historical classics, research evidence, and current prevention programs. Chin J Integr Med 1-8.
- World Health Organization (2020) News release: WHO welcomes preliminary results about dexamethasone use in treating critically ill COVID-19 patients.
- Zhou Y, Hou Y, Shen J, Huang Y, Martin W, et al. (2020) Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discovery 6:1-18.
- Xu X, Han M, Li T, Sun W, Wang D, et al. (2020) Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci 117:10970-10975.
- Fu B, Xu X, Wei H (2020) Why tocilizumab could be an effective treatment for severe COVID-19? J Transl Med 18:1-5.
- Cavalli G, De Luca G, Campochiaro C, Della-Torre E, Ripa M, et al. (2020) Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyper inflammation: a retrospective cohort study. The Lancet Rheumatology.
- Gabros S, Nessel TA, Zito PM (2019) Topical corticosteroids, in StatPearls [Internet]. StatPearls Publishing.
- Biggioggero M, Crotti C, Becciolini A, Favalli EG (2019) Tocilizumab in the treatment of rheumatoid arthritis: an evidence-based review and patient selection. Drug Des Devel Ther 13:57-70.
- Li Y, Liu X, Guo L, Li J, Zhong D, et al. (2020) Traditional Chinese herbal medicine for treating novel coronavirus (COVID-19) pneumonia: protocol for a systematic review and meta-analysis. Syst Rev 9:75.
- Neumann-Böhme S, Varghese NE, Sabat I, Barros PP, Brouwer W, et al. (2020) Once we have it, will we use it? A European survey on willingness to be vaccinated against COVID-19. Eur J Health Econ 21:977-982.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences