Clinical Evaluation of Volume Plethysmography as an aid for Diagnosis of Heart Failure in the Primary Care Setting
Scott C Howell1* and Robert C Master2
1Semler Scientific, Santa Clara, California
2Department of Interventional Cardiology, Stanford University School of Medicine, Stanford, California
Corresponding Author:
Received date: January 11, 2023, Manuscript No. IPJPM-23-15630; Editor assigned date:January 16, 2023, Pre QC No. IPJPM-23-15630 (PQ); Reviewed date: January 23, 2023, QC No. IPJPM-23-15630; Revised date:February 06, 2023, Manuscript No. IPJPM-23-15630 (R); Published date:February 27, 2023, DOI: 10.36648/2572-5483.8.1.184
Citation: Howell SC, Robert CM (2023) Clinical Evaluation of Volume Plethysmography as an Aid for Diagnosis of Heart Failure in the Primary Care Setting. J Prev Med Vol.8 No.1: 184.
Abstract
Approximately 6.2 million persons ≥ 20 years of age in the U.S have been diagnosed with Heart Failure (HF) and about 1 million new HF cases are diagnosed annually. HF remains primary diagnosis in >1 million hospitalizations annually, with the total cost of HF care in US exceeding $30 billion annually. Mortality rates after hospitalization for HF remain high; approximately 20%-25% within one year after diagnosis and mortality rates are similar for both HF with preserved Ejection Fraction (HFpEF) and HF with reduced Ejection Fraction (HFrEF). The most common way to diagnose HF is with transthoracic echocardiography (echo), which requires referral to a cardiologist and a cardiovascular technician. There is currently no low-cost method to identify HF, other than measuring Brain Natriuretic Peptides (BNP) in blood. QuantaFlo® HD (QFHD), a volume plethysmographic, point-of-care test performed by a medical aide in the primary care setting, presents real-time results to providers to aid in diagnosis of cardiovascular disease. Study objective was to measure the accuracy of QFHD as judged by echo determination of the diagnosis of HF.
Methods: QFHD and echo data were prospectively collected under an Institutional Review Board (IRB)-approved, multi- center, single-arm study. Test results for each technology were analyzed and used to design the algorithm to optimize accuracy. The algorithm was then prospectively validated in a second subject cohort from the same practices. Two separate metrics were computed and separate analyses were done to determine a diagnosis of HF. IRB approval of the study protocol was obtained through WIRB Copernicus Group (WCG) IRB. Written informed consent was obtained from each subject.
Results: A total of 414 subjects were enrolled in the first cohort used to create the algorithm. A total of 54 subjects were enrolled in second validation cohort. There was a significant correlation between QFHD and echo for diagnosing HF (p<0.01).
Conclusion: This study demonstrated QFHD is significantly correlated with echo for the diagnosis of HF in the primary care setting.
Keywords
HF; Cardiovascular disease; Preserved ejection fraction; Reduced ejection fraction; Left ventricular dysfunction; Valsalva maneuver; Transthoracic echocardiography; COVID-19 survivors; Asymptomatic heart dysfunction; Volume plethysmography system
Introduction
The prevalence rate of Heart Failure (HF) is estimated at 64 million people worldwide, of which approximately 6.2 million persons in the US ≥ 20 years of age have been diagnosed with HF and about 1 million new HF cases diagnosed annually with a prevalence that continues to rise [1]. The cost of HF is staggering with total annual healthcare expenditures exceeding $30 billion and over 1 million hospitalizations per year in the United States [2,3]. Mortality rates after hospitalization for HF remain high; approximately 20%-25% within one year of diagnosis and mortality rates for HF with preserved ejection fraction and HF with reduced ejection fraction are similar [4]. The re-admission rate for HF at six months approaches 50% [5-8]. The Center for Medicare and Medicaid Services (CMS) is currently penalizing hospitals financially for excessive 30-days re-admissions associated for HF.
Symptomatic heart failure (Table 1) is preceded by a prolonged asymptomatic stage in many patients. The number of patients with asymptomatic heart dysfunction is about 4-fold greater than the number of patients with clinically overt heart failure [9].
| Ages 45-54 | Ages 55-64 | Ages 65-74 | Over 75 | Total | ||
|---|---|---|---|---|---|---|
| Stage B (Asymptomatic) | (23.00%) | (32.30%) | (46.20%) | (39.00%) | (34.10%) | |
| Stage C (Symptomatic) | (2.20%) | (6.60%) | (14.40%) | (38.00%) | (11.80%) | |
Table 1: Prevalence of HF by age and stage.
Adapted from circulation 2007 prevalence and prognostic significance of HF stages application of the American College of Cardiology/American Heart Association HF Staging Criteria in the Community; Khawaja Afzal Ammar, MD.
HF represents a continuum from predisposing risk factors (stage A), asymptomatic but with structural changes (stage B), symptomatic (stage C) and advanced disease (stage D). Ejection fraction further defines HF with <40% as HFrEF (reduced), between 41%-49% HFmrEF (mildly reduced) and greater than 50% as HFpEF (preserved) [10]. Asymptomatic (stage B) HF can be a challenging clinical endeavor with suboptimal detection by primary care physicians despite significant prevalence.
The recent 2022 American College of Cardiology/American Heart Association (ACC/AHA) focused update for the Management of HF [11] and the 2021 European Society of Cardiology (ESC) guidelines for the diagnosis and treatment of acute and chronic heart failure [12] recommend screening with Brain Natriuretic Peptides (BNP) in peripheral blood for the at-risk population, (stage A, HF). The guidelines use a threshold in screening BNP of greater than or equal to 35 pg/ml to warrant subsequent cardiac referral and echocardiography (echo). While the use of screening BNP has high specificity for LV dysfunction, this test has low sensitivity [13].
Other new novel cardiac parameters have been incorporated into stage B HF definition with transthoracic echo utilizing calculation of left ventricular strain may be the best tool for diagnosis and characterization of heart dysfunction, but extensive use in the primary care setting is limited by availability and cost [14].
The ideal goal should be to identify and start prevention for these patients before they develop overt symptoms of HF (stage C) using cost-effective screening for asymptomatic heart dysfunction in at risk patients. Such interventions are an effective preventive approach in the asymptomatic stage to delay of clinical manifestations of HF. Neuroendocrine blockade therapy in asymptomatics with systolic LV dysfunction with beta-blockers and/or Angiotensin Converting Enzyme (ACE) inhibitors is reported to delay or prevent or delay the onset of symptoms and reduce mortality [9]. Neurohormonal antagonists, such as ACE inhibitors or angiotensin receptor blockers, have antihypertensive, anti-fibrotic and antithrombotic properties to reverse maladaptive remodeling developing in early-stage HF [15]. The Angiotensin Receptor-Neprilysin Inhibitors (ARNIs), beta blockers and Mineralocorticoids Receptor Antagonists (MRA) have been shown to reduce mortality in reduced ejection fraction HF [16, 17]. The cardiorenal axis is a new interventional target in heart failure with or without diabetes for Sodium-Glucose Cotransporter-2 inhibitors (SGLT2) with multiple international studies noting reduced cardiac composite endpoints of mortality and HF hospitalizations [7]. An echocardiographybased program designed to screen for early HF demonstrated an increase in guideline directed medical therapy in stage B HF for ACE inhibitors and beta blockers from 44%-77% and 15%-53%, respectively. Highlighting the problem of undertreating early HF, a European study showed only 22% and 12% appropriate titration of ACE inhibitors and beta blocker dosages over a three-month period as well as a higher risk of hospitalization or mortality, if 50% of the recommended dose was not achieved [18,19]. The early identification of stage B, HF provides an opportunity to delay or prevent progression to stage C. The Framingham study showed the prognostic risk of progression from asymptomatic Left Ventricular Systolic Dysfunction (LVSD) at entry resulted in a nearly 5-fold increase in the risk of developing symptomatic HF compared with those with normal LV function [20]. In communitybased observational studies, asymptomatic LVSD was associated with increased cardiovascular mortality, all-cause mortality and nonfatal cardiovascular events, such as myocardial infarction and stroke [21].
The increased prevalence of HF is another growing concern confronting primary care providers, particularly amid the massive burdens of COVID-19 infections and emerging data showing that COVID-19 survivors are more likely to develop a cardiovascular condition like HF or coronary disease over 30 days post infection, regardless of the severity of their COVID-19 infection [22]. Researchers warn that after COVID, health systems will likely see a significant increase in the number of patients with heart conditions related to the virus [23].
Given the collective impact of these issues confronting providers in primary care, it is important to develop an accurate, cost-effective; point-of-care testing that can be performed by a minimally trained medical aide with real-time results to aid the provider to be suspicious of the diagnosis of cardiovasculardisease. This study addresses the potential for QFHD to uncover early-stage HF in large, at-risk populations, in order to better target those that would benefit from echo and likely lead to Guideline Directed Medical Therapies (GDMT). The study objective was to measure the accuracy of QFHD as judged by ‘gold standard’ the echo determination of the diagnosis of HF.
Materials and Methods
From August 2020 through December 2021, three clinical practices (i.e., a family practice/urgent care center, a general cardiology practice and a heart failure clinic) prospectively enrolled subjects aged 45 years and older, excluding subjects with a pacemaker or contraindications to performing a valsalva maneuver. The first subject cohort was used to create the QuantaFlo algorithm. Then, the algorithm was prospectively validated in a second subject cohort from the same practices. Each subject was tested with QFHD and transthoracic echocardiography. Institutional Review Board approval of the study protocol was obtained through WIRB Copernicus Group (WCG) IRB. BNP was measured for subjects enrolled after May 2021.
Description of transthoracic echo
Echo is a routine diagnostic test, which is used to characterize cardiac function. The technician places an ultrasound probe at various points on the chest wall. This allows the echo equipment to create a model of the heart and compute various cardiac attributes including Left Ventricular Ejection Fraction (LVEF) and left ventricular Global longitudinal Strain (GLS). The echo equipment used in this study for computation of LVEF and GLS was GE Vivid™ iq. The operators were all registered through ARDMS with the credential of Registered Diagnostic Cardiac Sonographer (RDCS). The proprietary software automatically calculates both LVEF and GLS.
Criteria for HF diagnosis
One echo scan was done per subject from whom two separate metrics were computed (i.e., GLS and LVEF) and separate analyses were done to determine a diagnosis of HF using the following cutoffs:
• LVEF ≤ 45%
• GLS>-14%
QFHD measurements
Heart function has long been known to differ between normal hearts and weakened hearts when performing a valsalva [24-26]. Measurement with QFHD was performed by placing the sensor on a digit of the upper extremity. During an 8-second measurement, which included a valsalva maneuver, QFHD measured the blood volume changes in the finger. The results were then analyzed by an algorithm, which assessed function of the heart during the provocative maneuver (i.e., the valsalva). QFHD measurement was automatically graded by the algorithm as typical for HF or normal. The operators were blinded to the results.
BNP measurements
Blood samples were drawn and sent to a certified outside lab for BNP measurement. A cut-off of ≥ 35 pg/ml was used for screening for HF.
Statistical techniques
The Fisher’s exact test was used to evaluate the significance between the two categorical variables (i.e. QFHD vs. echo method). Statistical analysis was conducted using Statgraphics Centurion 19, version 19.2.02.
Results
A total of 414 de-identified subjects were enrolled in the first cohort used to create the algorithm. A total of 54 subjects were enrolled prospectively in second validation cohort, whose data are the basis of this report. A subset of 31 subjects from 54 subjects in validation cohort had BNP tests done. Subject population characteristics for the validation cohort are presented in the Table 2. Overall, the population had an average age of 62 years. Over half (76%) of the population tested was male.
| Demographics | Validation subjects | |
|---|---|---|
| All | with BNP | |
| Total | 54 | 31 |
| 46.30% | 45.20% | |
| HF by echo (GLS>-14%) | ||
| HF by echo (LVEF ≤ 45%) | 38.90% | 41.90% |
| Age (mean) | 62 years | |
| Gender (female) | 13 (24%) | 9 (29%) |
Table 2: Subject demographics.
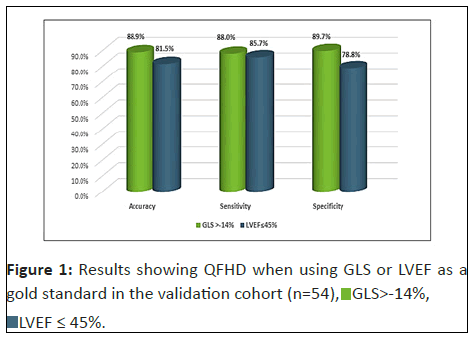
Using GLS as the gold standard for diagnosing HF, QFHD had an overall accuracy of 88.9%, a sensitivity of 88.0% and a specificity of 89.7% (Figure 1). There is a significant correlation between QFHD and GLS for diagnosing HF (p<0.01).
Using LVEF as the gold standard for diagnosing HF, QFHD had an accuracy of 81.5%, a sensitivity of 85.7% and a specificity of 78.8% (Figure 1). There is a significant correlation between QFHD and LVEF for diagnosing HF (p<0.01).
For the 31 subjects for whom BNP was measured: Using GLS as the gold standard for diagnosing HF, BNP had an accuracy of 61.3%, a sensitivity of 50.0%, and a specificity of 70.6% (Figure 2). Using GLS as the gold standard for diagnosing HF, QFHD had an accuracy of 90.3%, a sensitivity of 92.9% and a specificity of 88.2% (Figure 2). There is a significnt correlation between QFHD and GLS for subjects with BNP results for diagnosing HF(p<0.01).
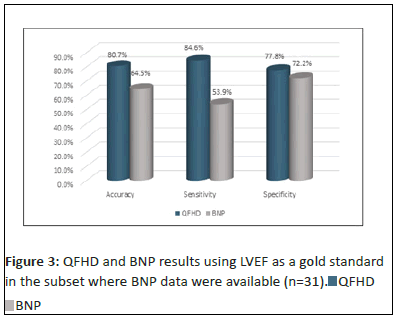
For the 31 subjects for whom BNP WAS measured: Using LVEF as the gold standard for diagnosing HF, BNP had an accuracy of 64.5%, a sensitivity of 53.9% and a specificity of 72.2% (Figure 3). Using LVEF as the gold standard for diagnosing HF, QFHD had an accuracy of 80.7%, a sensitivity of 84.6% and a specificity of 77.8% (Figure 3). There is a significant correlation between QFHD and LVEF for subjects with BNP results for diagnosing HF (p<0.01).
Discussion
This study examined the accuracy of a volume plethysmography system for the evaluation of heart failure using transthoracic echo (LVEF, GLS) as a ‘gold standard’. The QuantaFlo system has been shown to be accurate as an aid for diagnosing peripheral arterial disease [27]. In the study reported herein, an algorithm was employed to analyze the measurement of pulse recordings that were taken during a valsalva maneuver. The subjects prospectively enrolled were from a combined family practice/ urgent care center, a general cardiology practice and a heart failure clinic. The study findings were when using GLS as the criterion for HF, QFHD correlated with echo in 89% of cases (p<0.01) and, when using LVEF as the criterion for HF, QFHD correlated with echo in 82% of cases (p<0.01).
In the primary care practice, it is usually not practical to have a trained echocardiographer on staff or access to echocardiography equipment. Conversely, the QuantaFlo testing method may be accurately used by an office staff member with minimal training [26]. The system is automated and less subject to technique, settings, or manual calculations. Considering the reduced skill requirements, reliable ease of use and minimal time required, the QuantaFlo testing method may be well suited for the outpatient office setting as a screening tool to aid in the identification of patients more likely to benefit from an echo determination of HF.
Two well-accepted separate metrics were computed (GLS and LVEF) with separate analyses done with each to determine a diagnosis of HF. Of note, GLS is an assessment of myocardial fiber deformation that directly shown to be a more sensitive measurement of myocardial dysfunction than LVEF and is demonstrated in patients with HF with a reduced ejection to be a more powerful predictor of outcomes than LVEF [28-30].
Moreover, in a study of HF patients who normalized their LVEF to >50%, an abnormal GLS after recovery of LV function identified subgroups of patients who are more likely to undergo subsequent deterioration of LVEF, whereas a normal value for GLS identifies patients who are more likely to maintain a normal LVEF [31]. In a subset of subjects, BNP results were available. The ACC/AHA and ESC guidelines recommend the use of BNP to screen for HF withreference values ≥ 35 pg/ml for chronic HF [11,12]. In our study,using these threshold values, BNP results were shown to have lower accuracy than QFHD.
Limitations
Our study has several limitations. First, although the volume plethysmography screening test has promising accuracy, sensitivity and specificity metrics, severity categorizations remain to be documented. Second, the experience of the technique must be studied in larger general populations to better confirm our findings. Finally, follow-up with study of patient outcomes after GDMT when indicated have not yet been performed.
Conclusion
This study demonstrated the digital volume plethysmography system (QuantaFlo) is highly correlated with echo for the diagnosis of HF. This tool can be used by primary care physicians to screen large at-risk HF populations, in order to target those that would benefit to have an echo determination of HF and if indicated, guideline-directed medical therapies.
Acknowledgment
The authors would like to thank Semler Scientific for developing the parameters for the clinical study design. Dr. Douglas Murphy-Chutorian, MD, Semler Scientific, Inc. led the team that provided the conceptualization and design of the study, data collection and analysis. The authors confirm final editing responsibility for the following: Analysis, interpretation of results, manuscript preparation and final approval of the manuscript.
Declaration of Interest Statement
The authors report no conflict of interest other than Dr. Master had been a consultant to Semler Scientific, Inc. and Dr. Howell serves as Chief Innovation Officer at Semler Scientific, Inc.
Authors Contributions Statement
This author made substantial contributions to the conception or design of the work; he/she articulated and guided the interpretation of data for the work; and both drafted the verbiage and offered revisions that reflected important intellectual content, relevant background and information. Both provided final approval of the version to be published. Both agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data Availability Statement
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, such that supporting data is not available. Also, the authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
Declaration of Funding
Semler Scientific, Inc. sponsored this study. Dr. Master had been a consultant to Semler Scientific, Inc. Dr. Howell is CIO at Semler Scientific.
References
- Bozkurt B, Hershberger RE, Butler J, Grady K, Heidenreich PA, et al. (2021) ACC/AHA key data elements and definitions for heart failure: A report of the American College of Cardiology/American Heart Association task force on clinical data standards (writing committee to develop clinical data standards for heart failure). Circ Cardiovasc Qual Outcomes 14: e000102.
[Crossref], [Google scholar], [Indexed]
- Jackson SL, Tong X, King RJ, Loustalot F, Honget Y, et al. (2018) National burden of heart failure events in the United States, 2006 to 2014. Circ Heart Fail 11: e004873.
[Crossref], [Google Scholar], [Indexed]
- Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, et al. (2013) Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 6: 606-19.
[Crossref], [Google scholar], [Indexed]
- Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, et al. (2006) Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med 355: 260–269.
[Crossref], [Google scholar], [Indexed]
- James SL, Abate D, Hassen Abate K, Abay SM, Abbafati C, et al. (2018) Global, regional and national incidence, prevalence,and years lived with disability for 354 diseases and injuries for 195 countries and territories 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 392: 1789– 1858.
[Crossref], [Google scholar], [Indexed]
- Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, et al. (2009) 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines: Developed in collaboration with the international society for heart and lung transplantation. Circulation 119: e391–e479.
[Crossref], [Google scholar], [Indexed]
- Lam CS, Chandramoili C, Ahoola V, Subodh Verma (2019) SGLTâ?ÂÂÃÂ2 Inhibitors in heart failure: Current management, unmet needs and therapeutic prospects. J Am Heart Assoc 8: e013389.
[Crossref], [Google scholar], [Indexed]
- Loehr LR, Rosamond WD, Chang PP, Aaron RH, MPH, et al. (2008) Heart failure incidence and survival (from the atherosclerosis risk in communities study). Am J Cardiol 101: 1016–1022.
[Crossref], [Google scholar], [Indexed]
- Frigerio M, Oliva F, Turazza FM, Bonow RO (2003) Prevention and management of chronic heart failure in management of asymptomatic patients. Am J Cardiol 91: 4F-9F.
[Crossref], [Google scholar], [Indexed]
- Bozkurt B, Coats AJ, Tsutsui H, Abdelhamid M, Adamopoulos S, et al. (2021) Universal definition and classification of heart failure: A Report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail 21: S1071-S9164.
[Crossref], [Google scholar], [Indexed]
- Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, et al. (2022) 2022 AHA/ACC/HFSA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation 145: e876-e894. [Crossref],
[Google scholar], [Indexed]
- McDonagh TA, Metra M, Adamo M, Gardneret RS, Baumbach A, et al. (2021) ESC Scientific Document Group, 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. European Heart Journal 42: 3599-3726.
[Crossref], [Google scholar], [Indexed]
- McCullough PA, Nowak RM, James McCord J, Hollander JE, Herrmann HC, et al.(2002) B-type natriuretic peptide and clinical judgment in emergency diagnosis of heart failure analysis from Breathing Not Properly (BNP) multinational study. Circulation 106: 416-422.
[Crossref], [Google scholar], [Indexed]
- Shah AM, Claggett B, Loeh LR, Chang PP, Matsushita K, et al. (2017) Heart failure stages among older adults in the community the atherosclerosis risk in communities study. Circulation 135: 224–240.
[Crossref], [Google scholar], [Indexed]
- von Lueder TG, Kotecha D, Atar D, Hopper I (2017) Neurohormonal blockade in heart failure. Card Fail Rev 3: 19–24.
[Crossref], [Google scholar], [Indexed]
- Docherty KF, McMurray JJV (2019) Angiotensin receptor-neprilysin inhibitors: A new paradigm in heart failure with reduced ejection fraction. Int J Cardiol 281: 179-185.
[Crossref], [Google scholar], [Indexed]
- Yusuf S, Pitt B, Davis CE, Hood BH, Cohn JN, et al. (1991) Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 325: 293–302.
[Crossref], [Google scholar], [Indexed]
- Ghany R, Tamariz L, Chen G, Ghany A, Forbes E, et al. (2017) Screening echocardiograms in a senior focused value based primary care improves systolic heart failure detection and clinical management. Cardiovasc Diagn Ther 7: 236–243.
[Crossref], [Google scholar], [Indexed]
- Ouwerkerk W, Voors AA, Anker SD, Cleland JG, Dickstein K, et al. (2017) Determinants and clinical outcome of uptitration of ACEâ?ÂÂÃÂinhibitors and betaâ?ÂÂÃÂblockers in patients with heart failure: A prospective European study. Eur Heart J 38: 1883–1890.
[Crossref], [Google scholar], [Indexed]
- Kannel WB, Gordon T, and National Heart Institute (U.S.) (1968) The Framingham study: An epidemiological investigation of cardiovascular disease. Bethesda, MD., United States. Department of Health, Education, and Welfare, National Institutes of Health.
- Wang TJ, Levy D, Benjamin EJ, Vasan RS (2003) The Epidemiology of "asymptomatic" left ventricular systolic dysfunction: Implications for screening 138: 907-16.
[Crossref], [Google scholar], [Indexed]
- https://apps.who.int/iris/bitstream/handle/10665/332101/covid19-20200519.pdf?sequence=3&isAllowed=y
- https://medicine.wustl.edu/news/among-covid-19-survivors-an-increased-risk-of-death-serious-illness
- Zema MJ, Restivo B, Sos T, Sniderman, KW, Kline S, et al. (1980) Left ventricular dysfunction-the bedside valsalva manoeuvre revisited. Br Heart J 44: 560–9.
[Crossref], [Google scholar], [Indexed]
- Zema MJ, Caccavano M, Kligfield P (1983) Detection of left ventricular dysfunction in ambulatory subjects with the bedside valsalva maneuver.
[Crossref], [Google scholar], [Indexed]
- Zema MJ (1999) Diagnosing heart failure by the valsalva maneuver. Isn’t it finally time? JACC Heart Fail 116: 851–853.
[Crossref], [Google scholar], [Indexed]
- Diage TR, Johnson G, Ravipati G (2013) Digital ankle-brachial index technology used in primary care settings to detect flow obstruction: A population based registry study. BMC Research Notes 6: 404.
[Crossref], [Google scholar], [Indexed]
- Szymanski C, Levy F, Tribouilloy C (2014) Should LVEF be replaced by global longitudinal strain? Heart 100: 1655–1656.
[Crossref], [Google scholar], [Indexed]
- Smiseth OA, Torp H, Opdahl A, Haugaaet KH, Urheim S, al. (2016) Myocardial strain imaging: How useful is it in clinical decision making? Eur Heart J 37: 1196–1207.
[Crossref], [Google scholar], [Indexed]
- Sengelov M, Jorgensen PG, Jensen JS, Bruun NE, Olsen FJ, et al. (2015) Global longitudinal strain is a superior predictor of all-cause mortality in heart failure with reduced ejection fraction. JACC Cardiovasc Imaging 8: 1351–1359.
[Crossref], [Google scholar], [Indexed]
- Adamo L, Perry A, Novak E, et al. (2017) Abnormal global longitudinal strain predicts future deterioration of left ventricular function in heart failure patients with a recovered left ventricular ejection fraction. Circ Heart Fail 10: e003788.
[Crossref], [Google scholar], [Indexed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences
 GLS>-14%,
GLS>-14%,  LVEF = 45%.
LVEF = 45%.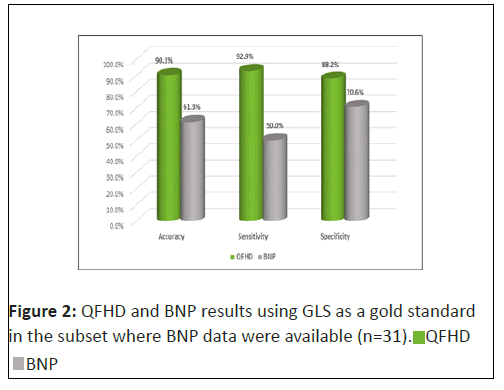
 BNP
BNP