Outbreak of Hospital Infection from Biofilm-embedded Pan Drug-resistant Pseudomonas aeroginosa, Due to a Contaminated Bronchoscope
Nader Alipour, Alper Karagoz, Abbas Taner, Nasrin Gaeini, Nastaran Alipour, Hassan Zeytin,Fatih Yildiz and Riza Durmaz
DOI10.21767/2572-5483.100014
Nader Alipour1*, Alper Karagoz2, Abbas Taner3, Nasrin Gaeini4, Nastaran Alipour5, Hassan Zeytin6, Fatih YÃÆââ¬Å¾ÃâñldÃÆââ¬Å¾Ãâñz1 and Riza Durmaz2
1Department of Microbiology & Biotechnology, Metu, Ankara, Turkey
2Department of Molecular Microbiology, Rafik Saydam Hifzi saha, Ankara, Turkey
3Department of Medical Microbiology, Kuru Hospital, Ankara, Turkey
4Department of Radiology, SÃÆââ¬Å¾ÃâðFA Medical Center, Gebze, Kocaeli, Turkey
5Department of Organic Chemistry, Tabriz University, Tabriz-Iran
6Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA
- *Corresponding Author:
- Nader Alipour
Department of Microbiology & Biotechnology
Metu, Ankara, Turkey
Tel: +90-554-6162952
Fax: +90-262-6412926
E-mail: nalipoure@yahoo.com
Received date: September 07, 2017; Accepted date: October 20, 2017; Published date: October 27, 2017
Citation: Alipour N, Karagoz A, Taner A, et al. (2017) Outbreak of Hospital Infection from Biofilm-embedded Pan Drug-resistant Pseudomonas aeroginosa, Due to a Contaminated Bronchoscope. J Prev Med Vol.2 No.2:1. doi: 10.21767/2572-5483.100014
Copyright: © 2017 Alipour N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Colistin-resistant Pseudomonas aeruginosa (P. aeruginosa) has been defined as pandrug-resistant (PDR) strain. Outbreaks of PDR P. aeruginosa especially in pulmonary tract infections due to contaminated bronchoscopes have rarely been reported. The emergence of pandrug-resistant strains in both CF (Cystic Fibrosis) and non-CF clinical isolates over recent years remains of a great concern. Hospital wards contaminated with PDR P. aeruginosa infection, must be shot down until their eradication. Health Authorities must be informed immediately and infection control strategies must be implemented.
Aim: To report such an outbreak and modify the infection control strategy in an academic hospital in Ankara Turkey.
Methods: From October to December 2013, PDRPseudomonas aerogionsa were identified from bronchial cultures of 15 patients who had undergone bronchoscopy prior to the infection. Three batches of surveillance cultures were obtained from the environmental objects and healthcare workers related to the procedures. Pulsed-field gel electrophoresis (PFGE) was used for bacterial typing. Antimicrobial susceptibility was assessed by disc diffusion and E-test methods.
Findings: A total of 70 specimens were obtained during the first surveillance operation. One Colistin-resistant P. aeroginosa was isolated from a bronchoscope. Although the disinfection protocols for bronchoscope were revised and implemented, seven additional bronchial cases were identified thereafter. The pathogen was identified from two subsequent surveillance cultures and was not eliminated until Ethylene oxide sterilization was added to the disinfection protocol. PFGE revealed that all 15 isolates from the patients and the three isolates from the bronchoscope shared a common pattern with minor variance. XbaI restriction enzyme turned out better than SpeI in interpreting bacterial pulse types with BioNumerics 6.0. The most suitable cut off value for SpeI was above 80% Dice similarity while for XbaI above 95%Dice similarity with BioNumerics 6.0.
Conclusion: The outbreak of “Colistin” pan drug-resistant Pseudomonas aeroginosa was caused by a contaminated bronchoscope and was terminated by the implementation of a revised disinfection protocol for bronchoscope.
Keywords
Pulsedfield gel electrophoresis; DNA finger printing; Pseudomonas aeruginosa; Bronchoscope; Nosocomial infection
Abbreviation
BAL: Bronchial Aspirate Lavage; ENT: Ear, Nose, Throat; ICU: Intensive Care Units; MDR: Multi Drug Resistant; PDR: Pan Drug- Resistant; ICPS: Infection Control Practitioners; OPA: Ortho- Phthalaldehyde; ETO: Ethylene Oxide; CF: Cystic Fibrosis
Introduction
Pandrug-resistance in Pseudomonas aeruginosa is defined by the US CF foundation consensus guidelines as resistance to colistin or all agents, and the emergence of pandrug-resistance (PDR) strains in both CF and non-CF clinical isolates over recent years remains of great concern [1].
Endoscopes are an important diagnostic and therapeutic instrument in modern medicine. However, if endoscopes were not subjected to a sufficiently high level of disinfection, they may cause outbreaks of healthcare-acquired infections (HCAIs). Almost all forms of endoscopes have been reported to be involved in outbreaks of HCAIs due to inadequate disinfection [2-5]. Among the different kinds of endoscopes, bronchoscope and gastrointestinal endoscopes have been most frequently reported as bearing pathogens. [6,7] Bacteria, Mycobacteria, fungi and viruses have all been reported as causing outbreaks [7-9]. However, outbreaks caused by Pan drug-resistance microorganisms on bronchoscopes have been rarely reported [7]. Colistin resistant Pseudomonas aeroginosa has been known as Pan drug-resistance bacteria. Due to life threatening of PDR Pseudomonas aeroginosa in old ages and immuno-comprmised patients, endoscopes must be precisely controlled time to time.
This article reports an outbreak of pulmonary tract infections caused by (Colistin) “PDR” Pseudomonas aerogionsa that had never been identified previously in Turkey’s hospitals. Laboratory investigation by molecular methods was performed and confirmed that this outbreak had been caused by a contaminated bronchoscope.
Methods
Turkey, Ankara “M” Hospital is a regional private, academic hospital in central Ankara, Turkey. The bronchoscopy department is operated by one attending doctor, bronchoscopy room is equipped with one rigid bronchoscope, and the bronchoscopy procedure is usually performed on an outpatient setting and an average of eight patients per week undergoes this procedure. The disinfection protocol for bronchoscope in the hospital was cleaning surface of bronchoscope tube with sterile gauze soaked in 70% ethanol or immersion of the scope in detergent/disinfectant solution. The method below was introduced by FDA-CDC,“1”, and we modified it as “2” [10-13].
1-Opening of all joints of the bronchoscopes, washing by tapwater, immersing in the diluted detergent containing enzymes (3M Rapid Multi-Enzyme Cleaner, including protease, amylase, lipase, and cellulose; 3M distilled water ¼ 1:100) for 15 min, and then immersion in 0.55% Ortho-phthalaldehyde (OPA) produced in last two weeks for 10 min (OPA strips were used prior to each immersion of broncoscope for confidence from its effectiveness, according manufacturer instruction). Final rinse with sterile distilled water was then executed to remove OPA residues.
2-Above protocol plus hypocholeric acid solution and ethylene oxide (ETO) weekly sterilization was added to the disinfection protocol for bronchoscope 15 minutes for each of them).
The outbreak
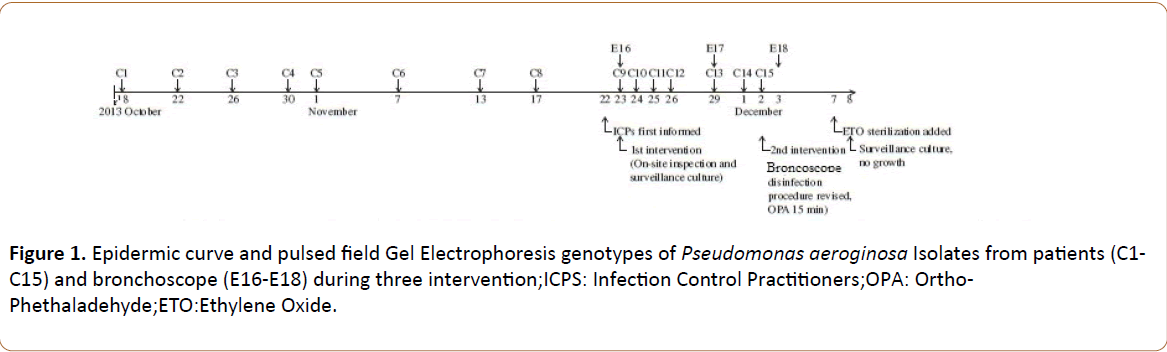
On 13 October 2013, one Colistin-resistant P. aeroginosa isolate was identified from the bronchial culture of a patient (case 1, Table 1) who developed bronchial infection after a bronchoscopy procedure in the bronchoscopy department. Similar isolates were subsequently identified from the bronchial specimens of the patients visiting the same department.
| Source | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | Case 10 | Case 11 | Case 12 | Case 13 | Case 14 | Case 15 | BSCOPE1 | BSCOPE2 | BSCOPE3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (Years) | 64 | 44 | 67 | 59 | 58 | 48 | 59 | 66 | 69 | 48 | 59 | 38 | 59 | 48 | 69 | |||
| Sex | M | F | F | F | F | M | M | M | M | M | M | M | F | M | M | |||
| Date of Broncoscopy | Oct-17 | 22-Oct | 26-Oct | 30-Oct | 01-Nov | 07-Nov | 13-Nov | 17-Nov | 23-Nov | 24-Nov | 25-Nov | 25-Nov | 26-Nov | 29-Nov | 01-Dec | 23-Nov | 29-Nov | 03-Dec |
Table 1. Demographics and clinical characteristics of the 15 cases with pulmonary tract infections owing to Pseudomonas aeroginosa and the sampling dates for the isolates from one bronchoscope (M=Male, F=Female)
On 13 November 2013, the microbiology staff informed the infection control practitioners of the emergence of this infection. The number of bronchial infection due to this microorganism had reached a total of seven cases. Once informed, the infection control practitioners immediately reviewed the medical charts of these seven patients, and found that all seven patients had had a bronchoscopy procedure before developing a bronchial infection.
Hence, an outbreak of PDR (Colistin-resistant) P. aeroginosa bronchial infection related to this procedure was highly suspected. Therefore, an investigation started on the following day.
On 13th November 2013, the first investigation was commenced and some interventions were conducted and PDR P. aeroginosa was isolated (described below); however, seven additional cases of bronchial infection caused by Colistinresistant P. aeroginosa were identified subsequently (29th November, the second infection control).
On 3rd December 2013, the third infection control interventions (described below) were conducted, and no new case was found. The date of these cases and the genotype of the isolates are shown in Table 1 and Figure 1.
Investigation and Interventions
The epidemiological curve of infected cases suggested exposure to a common source [9,10,14].
On-site inspection of the routine operation and disinfection procedures for the bronchoscopy found no inappropriateness. To identify the potential source of contamination, we conducted a surveillance culture for environmental objects and healthcare workers (HCWs) that were involved with the procedure of bronchoscopy.
The environmental objects included operating tables, taps, surfaces of washing tank, brushes of washing room, distilled water before and after rinsing, detergent enzyme for cleaning, hypochloric acid, OPA, bronchoscope and otoscopes, and their storage boxes. Samplings from the hands of HCWs involved in the bronchoscopy procedure were also obtained. A total of 70 samples were taken, comprising 48 samples from the environment, 17 samples from instruments, and 5 samples from the hands of HCWs.
All specimens from the surfaces of the suspected subjects described above, including the hands of HCWs, were obtained by swabbing the surface with sterile cotton swabs immersed in culture broth. For each scope, three specimens were taken. The surfaces of the eye piece and joint (after being released from the scope) were sampled as described above. The external channel of the scopes was flushed with sterile distilled water, which was concentrated by centrifugation and cultured.
Liquid samples that contained antimicrobial substances were processed with a 0.45 μ filter and the dregs were cultured; the other suspected fluids were centrifuged and cultured.
All of the instruments associated with the procedure of bronchoscopy were thoroughly cleaned and disinfected. The enzyme solution used for cleaning, despite manufactory guideline about OPA reusability till two weeks, for minimizing our research variables the OPA for disinfection were discarded and fresh solutions provided. The personnel responsible for the disinfection of bronchoscope were asked to implement the disinfection policy strictly. At this point, the bronchoscope was in use as usual.
Sterilization of bronchoscopes in details
Sterilization in the surgical field has been the primary modality in preventing spread of infections. We followed Igor Naryzhny Sterilization method [15]. As such, we proceeded to change reprocessing of our Olympus bronchoscope from automated high-level disinfection (HLD) to combined HLD followed by ETO gas sterilization of all bronchoscopes, as well as additional pre-cleaning steps. To help reduce visible bio-burden, each bronchoscope was pre-cleaned (First Step Bedside Pre- Clean Kit; Cygnus Medical, Branford, Conn) immediately after patient use before transport for reprocessing. The bronchoscope was then brought to the cleaning room where it was manually cleaned with multi-enzyme detergent/cleaner (CST-404C Surg- ENZ; Moorestown, NJ) using manufacturer recommendations. After this, the bronchoscope was placed into a washer/ disinfector for reprocessing (System 83 Plus; Custom Ultrasonics, Ivyland, Pa) using a high level disinfectant (MetriCide OPA Plus; Metrex Research, Orange, Calif). All recommendations and guidelines provided by the endoscope manufacturer, as well as the manufacturers of the cleaning/disinfecting solutions and equipment, were closely followed. After HLD, the bronchoscope was then brought to the surgical sterilization facility. The Pentax proposed guidelines for ETO gas sterilization of bronchoscopes were closely followed along with, as suggested by Pentax, the guidelines of the sterilizer manufacturer. Igor Naryzhny institution had already equipped with 4 dual-cycle 100% ethylene oxide gas sterilizers (Steri-Vac Sterilizer/Aerator 8XL; 3M, St. Paul, Minn) for use on surgical equipment including endoscopes used during surgical procedures. Guidelines recommended by the Association for the Advancement of Medical Instrumentation (AMMI) as well as the manufacturers of the equipment were strictly followed. Hypochloric acid sterilization was fulfilled for 15 minutes as our novel method. Each bronchoscope was placed into a disposable wrap (Halyard; Alpharetta, GA), then placed into the sterilizer chamber along with surgical equipment. The sterilization process consisted of machine startup, a 1-hour automated sterilization with 3M 100% ETO single-use cartridge and a 12-hour aeration cycle, totaling at least 15 to 16 hours (this did not include HLD time). The bronchoscope was then brought to the bronchoscopy department where it was removed from the sterilized wrapper and visually inspected. If not immediately used, the bronchoscope was hung in a non-sterile storage cabinet (Model 20000; Custom Ultrasonics, Ivyland, Pa) with medical air aeration.
If the bronchoscope was not used in 5 days, it underwent reprocessing with HLD as described above. Due to lack of guidelines, we began culturing each bronchoscope specifically for PDR on a monthly basis 4 months after initiating the sterilization process. Culturing of all bronchoscopes was performed at the same time, therefore a bronchoscope may have been cultured either after HLD but before ETO sterilization, immediately after ETO sterilization or any time after ETO or additional HLD before its use; (I, timing of culture was random within each bronchoscope sterilization cycle) As previously identified as the culprit for contamination and potential nidus for organic debris, the elevator mechanism of the bronchoscope was cultured in the up and down position (ESwab Collection Kit; ACL Laboratories, Rosemont, Ill). Culturing was performed under sterile technique, which included disinfection of the counter and the use of gowns, face masks/shields, hair covering, and sterile gloves. After appropriate preparation, the outer tip of the bronchoscope was sanitized with an alcohol pad using caution to not wipe the elevator mechanism and lens face at the distal end that was to be sampled with the ESwab; the bronchoscope was air dried before sampling. The bronchoscope was placed in a tray with sterile pad/liner for sampling. The ESwab was dipped in the medium of the transport container to pre-moisten, and excess fluid was pressed from the ESwab inside the inner walls of the container. The ESwabs were held above the red mark on the shaft as that was the part of the shaft that was broken off and discarded; the shaft below the red mark was not touched. One ESwab was used for both the up elevator and the down elevator positions. The inside of the elevator mechanism, recess and channel in the down position were sampled. Next, the same ESwab was used to sample the elevator mechanism and recess in the up position, as well as to scrub the face of the lens. The ESwab was then placed into the transport tube, which was tightened and labeled as indicated. After culturing, the specimen was sent to ACL Laboratories and the bronchoscope was reprocessed with HLD. At that time, awaiting the results of cultures did not preclude the use of the bronchoscopes [15].
Laboratory investigations
All the clinical isolates of P. aeroginosa as well as those from surveillance cultures were identified by standard methods of the Clinical and Laboratory Standards Institute (CLSI), and further characterized by molecular methods. Antibiotic susceptibility testing was done by two methods, namely disc diffusion and Etest. Susceptibility to the following antibiotics was tested using a disc method: Carbenicillin (Cb), piperacillin-tazobactam (Tzp), ceftazidime (Caz:30 mg), imipenem (Imp:10 mg), meropenem (Mem:10 mg), gentamicin (Gn:10 mg), tobramycin (Tb), netilmicin (Net), amikacin (Ak:30 mg), ciprofloxacin (Cip), colistin (Ks: 8 mg), minimum inhibitory concentrations (MICs) of ertapenem, imipenem, meropenem and colistin were also assessed with E-test strips (AB Bio disk, Bio Merieux). Interpretation was according to CLSI breakpoint concentrations (CLSI guidelines M100S26) [11].
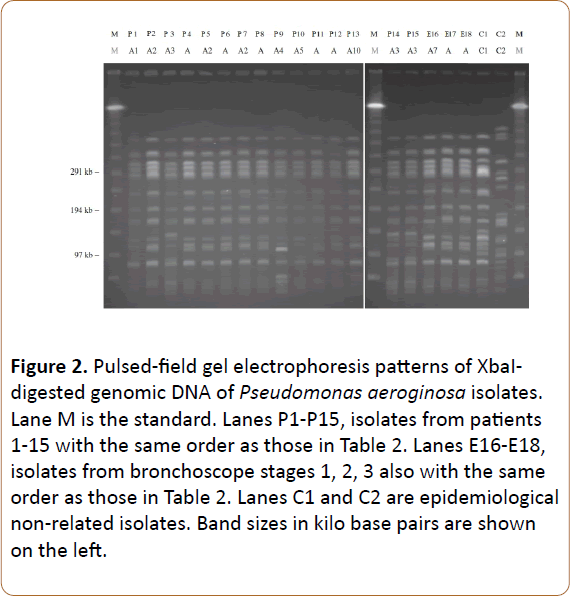
Pulsed-field gel electrophoresis (PFGE) of the XbaI, SpeI (New England Bio labs, Beverly, MA, USA) macro-restricted genomic DNA, was performed to delineate the genetic relatedness of the isolates using procedures described previously [12,16]. PFGE patterns were interpreted according to the criteria suggested by Tenover et al. and the genotypes were designated in alphabetical order [17]. PFGE patterns with one to three band differences from an existing genotype were defined as subtypes of that genotype and were labeled with Arabic number suffixes. Two isolates were considered to be indistinguishable, highly related, or distinct if they had the same subtype (no band difference), the same genotype (one to three band differences), or different genotype (four or more band differences), respectively. Two epidemiologically non-related P. aeroginosa isolates (Figure 2, C1 and C2) were used as control “Marker” strains.
Figure 2: Pulsed-field gel electrophoresis patterns of XbaIdigested genomic DNA of Pseudomonas aeroginosa isolates. Lane M is the standard. Lanes P1-P15, isolates from patients 1-15 with the same order as those in Table 2. Lanes E16-E18, isolates from bronchoscope stages 1, 2, 3 also with the same order as those in Table 2. Lanes C1 and C2 are epidemiological non-related isolates. Band sizes in kilo base pairs are shown on the left.
Biofilm detection
Biofilm existing was evaluated by two laboratory methods plus electron microscopy method as follows.
ATP consumption detection by bio-luminesance
Final washing solutions at the end of each interventional stage was used for evaluating presence or absence of living bacteria, according to protocol of BioThema Isogen (Product number 266-11).
Crystal violet method
By above method, final washing water of three interventional stage was used for evaluating biofilm formation as follow; after washing with distilled water, 100 micro-liter of washing water was place in micro-plate and air fixed, micro-plate was stained with 110 micro-liter of 0.4% aqueous crystal violet solution for 45 minutes, after wards each well was washed four times with 350 micro-liter of sterile distilled water and immediately destined with 200 micro liter of 95% ethanol. After 45 minutes of distaining, 100 micro liter of destining solution was transferred to a new well and destining solution was measured with ELISA reader (Labile ER 2007 Micro plate washer, DIAGNOVA) at 595 nm.
Scanning Electron Microscopy
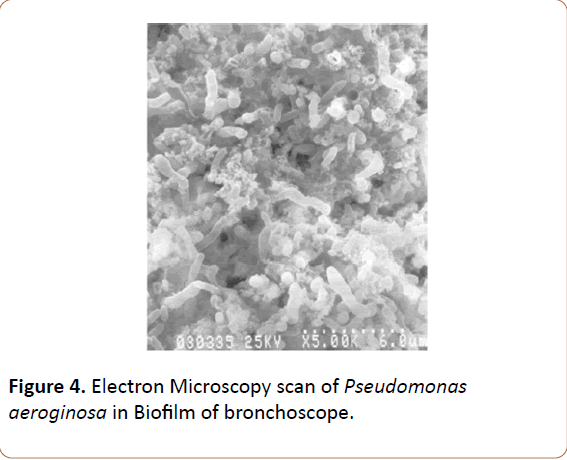
SEM was performed following the method of Hawser et al [18]. Briefly, P. aeruginosa biofilms that were formed on bronchoscope external channel surface were scratched with bistury blade and transferred on pieces of PVC (1.0 cm2) and were fixed with 2.5% (v/v) glutaraldehyde in PBS for 5 h at room temperature. The samples were then washed with PBS and dehydrated in an ethanol series (30, 50, 70, 90, and 100%). All of the samples were subsequently dried overnight, gold coated and viewed under SEM (Zeiss EVO MA 5).
Results
In epidemiological investigation and management results from the surveillance culture, one Colistin-resistant Pseudomonas aeroginosa isolate was identified from the external channel of the most frequently used bronchoscope. Although the infection control interventions described above were implemented.
Seven additional cases of bronchial infection caused by Colistin-resistant P. aeroginosa were still identified subsequently. To clarify the source of the contamination, on 29th November 2013, the second surveillance culture was performed on the frequently used bronchoscope. Of the six samplings obtained, one Colistin-resistant Pseudomonas aeroginosa isolate was again identified from the external channel of the contaminated bronchoscope. During this period, the infection control practitioners also identified that this specific bronchoscope had been used on all the infected patients before they developed the bronchial infection. Therefore, on 2nd December 2013, the bronchoscope was suspended from use. In addition, because the original disinfection procedure was apparently unable to decontaminate the Colistin-resistant P. aeroginosa from bronchoscope, the whole protocol was revised as follows: opening of all joints of bronchoscope, washing with tap-water, irrigating the external channel of bronchoscope with diluted enzyme (newly added),bronchoscope immersed in the diluted enzyme for 15 min, irrigating external channel of bronchoscopes with 0.55% OPA (freshly made daily), and then immersion of the bronchoscope in 0.55% OPA for 15 min (extended for 15 min),followed by thoroughly rinsing with sterile distilled water. On 3rd December 2013, the third surveillance culture of the contaminated bronchoscope was performed.
Again, Colistin-resistant P. aeroginosa was isolated from the external channel of the bronchoscope. Then, weekly ethylene oxide (ETO) sterilization was added to the disinfection protocol for bronchoscopes [15]. On 8th December 2013, the fourth surveillance culture of the contaminated bronchoscope was done, and no microorganism was identified this time. Since then, the bronchoscope has been re-used again, and no more cases of bronchoscopy-related bronchial infection have been found. Above results were confirmed by our biofilm evaluating tests. Biofilm embedded living bacteria was existed till third infection control interventional stage (Figures 1-3 and Table 3).
Results of antibiotic susceptibility testing are shown in Table 2. According to the disc diffusion method, most isolates were resistant to routinely used antibiotics (94%). According to the Etest, most isolates were resistant to gentamicin and cephalosporins, and all isolates were sensitive to aztreonam.
| Isolate | PFGE Genotype | Antibiotic Susceptibility Testing | MIC (µg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AMI | GM | COL | CRO | CAZ | IPM | ERT | IPM | MER | ERT | ||
| Case 1 | A1 | S | S | R | I | R | S | S | 0.5 (S) | 0.125 (S) | 2.0 (S) |
| Case 2 | A2 | S | R | R | R | R | S | I | 1.0 (S) | 1.0 (S) | 4.0 (I) |
| Case 3 | A3 | S | R | R | R | R | S | R | 2.0 (S) | 2.0 (S) | 16 (R) |
| Case 4 | A | S | R | R | R | R | S | I | 2.0 (S) | 2.0 (S) | 4.0 (I) |
| Case 5 | AZ | S | R | R | R | R | S | I | 1.0 (S) | 1.0 (S) | 4.0 (I) |
| Case 6 | A | S | R | R | R | R | S | I | 1.0 (S) | 1.0 (S) | 8 (R) |
| Case 7 | AZ | S | R | R | R | R | S | R | 4.0 (S) | 2.0 (S) | 16 (R) |
| Case 8 | A | S | R | R | R | R | S | R | 2.0 (S) | 0.125 (S) | 32 (R) |
| Case 9 | A4 | S | R | R | S | S | S | I | 0.2 (S) | 0.5 (S) | 1.0 (S) |
| Case 10 | A5 | S | R | R | I | R | S | S | 1.0 (S) | 1.0 (S) | 0.5 (S) |
| Case 11 | A | S | R | R | R | R | S | R | 2.0 (S) | 1.0 (S) | 8 (R) |
| Case 12 | A | S | R | R | R | R | I | R | 4.0 (S) | 2.0 (S) | 16 (R) |
| Case 13 | A10 | S | R | R | R | R | S | R | 4.0 (S) | 2.0 (S) | 16 (R) |
| Case 14 | A3 | S | R | R | R | R | S | R | 2.0 (S) | 2.0 (S) | 8 (R) |
| Case 15 | A3 | S | R | R | R | R | S | R | 4.0 (S) | 2.0 (S) | 12 (R) |
| Scope 1 | A7 | S | R | R | R | R | S | I | 2.0 (S) | 1.0 (S) | 4.0 (I) |
| Scope 2 | A | S | R | R | R | R | S | I | 2.0 (S) | 1.0 (S) | 8 (R) |
| Scope 3 | A | S | R | R | R | R | S | R | 2.0 (S) | 2.0 (S) | 32 (R) |
Table 2. Laboratory investigation of the 18 Pseudomonas aeroginosa isolates analyzed
PFGE results revealed that all 15 isolates from the patients and three isolates from the bronchoscope shared a common pattern but several subtypes were identified, whereas the two non-related isolates showed different patterns (Figure 4).
MIC of Colistin resistance was determined 8 mg/l, this MIC level has been defined colistin resistant by all known standard systems (Table 4).
| Pseudomonas aeroginosa | Acinetobacter baumannii | Enterobacteriaceae | |||||
|---|---|---|---|---|---|---|---|
| S | I | R | S | R | S | R | |
| EUCAST | ≥ 4 | - | > 4 | ≥ 2 | > 2 | ≥ 2 | > 2 |
| CLSI | ≥ 2 | ≥ 4 | ≥ 8 | ≥ 2 | ≥ 4 | - | - |
| BSAC | ≥ 2 | - | ≥ 8 | - | - | ≥ 4 | ≥ 8 |
| SFM | ≥ 2 | - | > 2 | ≥ 2 | > 2 | ≥ 2 | > 2 |
Table 4. Minimum inhibitory concentration (MIC) amount of colistin resistant bacteria in different standard system (mg/L)
Results of ATP consumption and Biofilm formation detection by Scanning Electron Microscopy and Crystal Violet testing are shown in Table 3.
| 14 November First infection control stage | 29 November Second infection control stage | 3 December Third infection control stage | |
|---|---|---|---|
| ATP consumpion test | Positive (+) | Positive (+) | Negative (-) |
| Crystal Violet Test | Positive (+) | Positive (+) | Negative (-) |
| Scanning Electron Microscopy | Positive (+) | Positive (+) | Negative (-) |
Table 3. Biofilm detecting test results in 3 infection control stages
According to the above methods Biofilm embedded microorganisms had remained “living” form until using ETO. After ETO application (third interventional stage “E18”) above test results became negative. These results show, FDA-CDC confirmed methods are merely effective on planktonic forms of bacteria and they are non-effective against biofilm embedded forms. According to above tests, ATP consumption remains positive till (E18).
Discussion
To our knowledge, this is the first report of an outbreak of pan drug-resistant P. aeroginosa due to a contaminated bronchoscope in turkey. All the 15 cases of bronchial infection in this outbreak occurred at the outpatient department where the patients received bronchoscopy. Even though two-thirds of the patients were hospitalized for treatment,
None of them was categorized as having bronchial infection initially. The outbreak therefore elucidated the infection control surveillance system. In addition, the attending bronchoscopy, pulmonary doctors were not alert enough to recognize, or even suspect on the cluster of these cases and probability of nonefficiency of CDC-FDA recommended sterilization procedure on biofilm embedded bacteria. Hence, the outbreak was not identified until the microbiology staff informed the infection control practitioners. Again, it cannot be overemphasized that the responsibility of infection control is not limited to certain personnel but ascribed to all the personnel working in the hospital. All HCWs in the hospital should receive continuous medical education on infection control issues regularly. All 15 clinical isolates were identified as resistant to Colistin by the disc diffusion test initially and MIC later on. 8 mg/l was MIC of all isolated Pseudomonas aeroginosa, which has been defined resistant by all known standard systems (Table 4). However, all 18 isolates (15 from the patients, 3 from the bronchoscope) involved in the outbreak shared a common PFGE pattern, Although several subtypes were also identified Moreover, the contaminated bronchoscope had been used on all 15 patients before they developed the bronchial infection, while no new case of bronchial infection caused by Colistin-resistant P. aeroginosa was further identified after suspension of the contaminated bronchoscope. These results suggest that this event was an outbreak of Colistin-resistant P. aeroginosa bronchial infection, and the contaminated bronchoscope was the cause of the outbreak.
A recent report from a nearby university-affiliated hospital indicated that the prevalence rate of Colistin resistance among clinical samples of P. aeroginosa isolates was 7% [19].
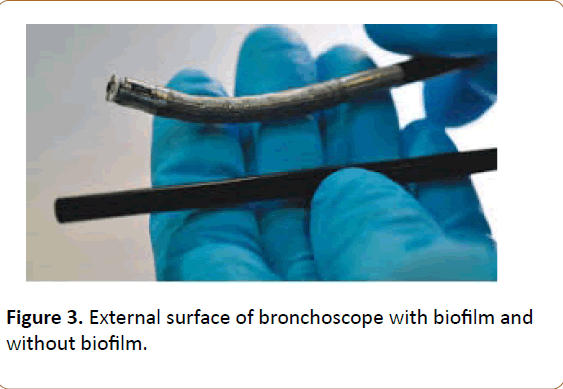
Isolates of Colistin-resistant P. aeroginosa identified in this outbreak might be from clonal spread rather than new mutants. Resistance patterns of P. aeroginosa isolates should be continuously monitored, and further studies should be performed to evaluate whether the clone had spread in this area. During the intervention of this outbreak, we did not uncover the reason why the OPA disinfection protocol could not thoroughly eradicate P. aeroginosa until ETO sterilization was added. We speculate that this should be due to biofilm formation around bacteria in bronchoscope that prevents the penetration of the disinfectant material from biofilm barrier and penetration of ETO from that barrier. Addition of ETO apparently broke this barrier. Our biofilm detecting methods confirmed existing biofilm and living bacteria till using ETO for their sterilizations (Table 4). Olympus Bronchoscope experts confirmed our idea as they had similar experience with the similar equipment. Their electron microscopic study also confirmed biofilm e xis t ence in our br onchosc ope (Figure 3-5).
Previous studies indicated that the most usual factors for disinfection failure, which also led to endoscopy-related outbreaks, included equipment malfunction, inadequate disinfection practice, and contamination of washer, accessories, water or other solutions used [6,7] to elucidate whether these factors were associated with the outbreak presented here, the following points have been examined or improved during the investigation. The concentration and the immersion time of the 3M diluted enzyme used were both above the highest requirements recommended by the manufacturer. In addition, the immersion time of 0.55% OPA was changed from 10 to 15 min that is longer than the 12 min recommended by the US Center for disease control and US Food and Drug Administration. Other factors, such as contaminated water or solutions, were not identified by surveillance cultures. However, the OPA disinfection, with either the initial or the revised protocols, indeed failed to decontaminate bronchoscope. Two possibilities may explain this disinfection failure:
First, the outbreak isolates might have generated biofilms in the bronchoscope, leading to the disinfection failure [17,20]. OPA is unable to destroy the microorganisms within biofilms, and, worst of all, may facilitate the accumulation of biofilms [18,21]. A heat-sterilization procedure, such as autoclave, is more effective in eliminating the microbial biofilms. However, because some elements in the endoscopes may be vulnerable to high temperatures, ETO is suggested as one of the alternative methods for endoscope disinfection [19,22]. Indeed, by adopting the ETO procedure, we successfully decontaminated bronchoscope.
Second, a gene mutation might have been occurred, resulting in reduced susceptibility or even full resistance of the microorganism to OPA [23-25]. It has been shown that decreased porin expression played a major role in the resistance to aldehyde-based disinfectants [23,26]. A recent report from an Asian hospital indicated that decreased expression of outer membrane proteins (porins) is one of the important factors contributing to the aldehyde resistance of Gram negative rods [16,27].
Although we did not examine the OPA resistance among our outbreak isolates, these bacteria may also possess the same characteristics that contribute to the Colistin resistance and therefore to the OPA resistance. Further studies are needed to elucidate these issues.
Retrospectively, we recognized that one mistake had been made in managing this outbreak. When P. aeroginosa was identified from the bronchoscope on the first surveillance, we did not suspend the usage of this contaminated bronchoscope immediately, though we had presumed that the contaminated bronchoscope might not be the cause of this outbreak. Continuing usage of the contaminated bronchoscope led to further cases of infection.
This clearly taught us that, once identified, a contaminated instrument should be suspended and not used until the potential pathogen has been eradicated or proved otherwise. Again, to prevent endoscopy-related infections, strict implementation of the disinfection protocol of endoscopes cannot be overemphasized [7,24,25,28].
In conclusion, the outbreak of colistin-resistant P. aeroginosa bronchial infection was due to the disinfection failure of a contaminated bronchoscope, which had been colonized by the resistant clone probably after being used on the case 1 patient. Infection after bronchoscopy or other endoscopic procedures should be closely monitored. If two consecutive or more patients develop infections after the procedure, especially if the same clinically relevant microbial species are involved, the existence of an outbreak of infection should be highly suspected and a prompt investigation should be initiated. Furthermore, if scopes are found to be contaminated or colonized by a resistant pathogen that may possess characteristics
Similar to the Colistin-resistant P. aeroginosa described herein, the deficiency of OPA disinfection should be considered and more effective decontamination strategies should be considered. Before the sterility can be ensured, further use of the contaminated scopes should be prevented.
Acknowledgement
The authors thank the staff in the bronchoscopy, pulmonary department, clinical microbiology laboratory and molecular microbiology department for their co-operation and technical support during the outbreak investigation.
The authors would also like to thank prof. MA Rafi, from Thomas Jefferson University, Philadelphia, USA for his valuable advice and editorial assistance.
The authors would also like to thank Nastaran Alipour, (PhD student of chemistry) from Tabriz University, for correcting reference section of this manuscript with Endnote software and her editorial assistance. This work was supported in part by BAP project Grant from Rafik saydam hÃÆââ¬Å¾ÃâñfzÃÆââ¬Å¾Ãâñ saha institute (Turkey National Institute of Public Health) and part by Grant 1R15AI089671-01from the National Institute of Health.
There is not any conflict of interest between co-authors of this manuscript.
Ethical Issues
There is not any Ethical issue in this research.
Conflict of Interest
There is not any conflict of interest between co-authors of this manuscript.
Study Highlights
What is current knowledge?
1- The most standard and recommended disinfection protocol for bronchoscope in the hospital was FDA-CDC guideline method; it is effective on non-biofilm embedded forms of bacterias. The routine disinfection protocol for bronchoscope in the hospital was cleaning surface of bronchoscope tube with sterile gauze soaked in 70% ethanol or immersion of the scope in detergent/disinfectant solution.
2- XbaI and SpeI restriction enzymes has been used for Pulse Field Gel Electrophoresis based on epidemiological studies for Pseudomonas aeroginosa outbreaks in health centers, they were fulfilled without evaluating their cut of value (Coefficient similarity) and their priority and efficiency.
What is new here?
1-These results show, FDA-CDC confirmed methods are merely effective on planktonic forms of bacteria and they are non-effective against biofilm embedded bacterias, this guideline must be uptodated.
2- XbaI restriction enzyme turned out better than SpeI in interpreting bacterial pulse types with BioNumerics 6.0. The most suitable cut off value for SpeI was above 75% Dice similarity while for XbaI above 95% Dice similarity with BioNumerics 6.0, So Using XbaI is recommended, PFGE relevant protocol must be uptodated.
References
- Armstrong DS, Nixon GM, Carzino R, Bigham A, Carlin JB, et al. (2002) Detection of a widespread clone of Pseudomonas aeruginosa in a pediatric cystic fibrosis clinic. Am J Respir Crit Care Med 166: 983-987.
- Aumeran C, Poincloux L, Souweine B, Robin F, Laurichesse H, et al. (2010) Multidrug-resistant Klebsiella pneumoniae outbreak after endoscopic retrograde cholangiopancreatography. Endoscopy .Thieme Publishing Group 42: 895-9.
- Corne P, Godreuil S, Pierre JH, Jonquet O, Campos J, et al. (2005) Unusual implication of biopsy forceps in outbreaks of Pseudomonas aeruginosa infections and pseudo-infections related to bronchoscopy. J Hosp Infect 61: 20-26.
- Wendelboe AM, Baumbach J, Blossom DB, Frank P, Srinivasan A, et al. (2008) Outbreak of cystoscopy related infections with Pseudomonas aeruginosa: New Mexico, 2007. J Urol 180: 588-592.
- Muscarella LF (2008) Reassessment of the risk of healthcare-acquired infection during rigid laryngoscopy. J Hosp Infect 68: 101-107.
- Spach DH (1993) Transmission of infection by gastrointestinal endoscopy and bronchoscopy. Ann Intern Med 118: 117-128.
- Vazquez SE, Monguio RR, Visaria J, Carlson A (2006) Exogenous endoscopy-related infections, pseudo-infections, and toxic reactions: clinical and economic burden. Curr Med Res Opin 22: 2007-2021.
- Ramsey AH, Oemig TV, Davis JP, Massey JP, Toöroök TJ, et al. (2002) An outbreak of bronchoscopy-related Mycobacterium tuberculosis infections due to lack of bronchoscope Leak Testing. Chest 121: 976-981.
- Rey JF, Halfon P, Feryn JM, Khiri H, Masseyeff MF, et al. (1995) Risk of transmission of hepatitis C virus by digestive endoscopy. Gastroenterol Clin Biol 19: 346-349.
- Patterson P (2009) CDC sterilization, disinfection guideline. OR Manager 25: 14.
- Food, Administration D, Control CfD, Prevention. FDA and CDC public health advisory (1999) Infections from endoscopes inadequately reprocessed by an automated endoscope reprocessing system. Food and Drug Administration .
- Rutala WA, Weber DJ (2010) Guideline for disinfection and sterilization of prion-contaminated medical instruments. Infect Control Hosp Epidemiol 31: 107-117.
- Bucx MJ, Dankert J, Beenhakker MM, Harrison TE (2001) Decontamination of laryngoscopes in the Netherlands. Br J Anaesth 86: 99-102.
- Bennett JV, Jarvis WR, Brachman PS (2007) Bennett & Brachman's hospital infections. Lippincott Williams & Wilkins 5.
- Naryzhny I, Silas D, Chi K (2016) Impact of ethylene oxide gas sterilization of duodenoscopes after a carbapenem-resistant Enterobacteriaceae outbreak. Gastrointest Endosc 84: 259-262.
- Su LH, Leu HS, Chiu YP, Chia JH, Kuo AJ, et al. (2000) Molecular investigation of two clusters ofhospital-acquired bacteraemia caused by multi-resistant Klebsiella pneumoniae using pulsed-field gel electrophoresis andinfrequent restriction site PCR. J Hosp Infect 46: 110–117.
- Tenover FC, Arbeit RD, Goering RV (1995) Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol 33: 2233-2239.
- Hawser SP, Baillie GS, Douglas LJ (1998) Production of extracellular matrix by Candida albicans biofilms. J Med Microbiol 47: 253-256.
- Aktepe OC, Çetinkaya Z, HakkÃÆââ¬Å¾Ãâñ Çiftçi ÃÆââ¬Å¾Ãâð, AÃÆââ¬Â¦Ãâà ¸ÃÆââ¬Å¾Ãâñk G (2009) The antibiotic resistance of Pseudomonas aeruginosa strains isolated from various clinical specimens. Pathology 41: 77.
- Pajkos A, Vickery K, Cossart Y (2004) Is biofilm accumulation on endoscope tubing a contributor to the failure of cleaning and decontamination? J Hosp Infect 58: 224-229.
- McDonnell G, Russell AD (1999) Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev 48: 613-615.
- FrÃÆââ¬Å¾ÃâÃâÃÆââ¬Â¦ÃâãilÃÆââ¬Å¾ÃâÃâ O, TanÃÆââ¬Â¦ÃâãÃÆââ¬Å¾ÃâÃâu M (2006) Cleaning and disinfection in gastrointestinal endoscopy: current status in Romania. J Gastrointestin Liver Dis 15: 89-93.
- Sheldon AT (2005) Antiseptic “Resistance”: Real or Perceived Threat? Clin Infect Dis 40: 1650-1656.
- Cabrera HR, Alcaide VM, Aceñero FM (2004) The influence of laboratory adaptation on test strains, such as Pseudomonas aeruginosa, in the evaluation of the antimicrobial efficacy of ortho-phthalaldehyde. J Hosp Infect 57: 217-222.
- Fisher CW, Fiorello A, Shaffer D, Jackson M, McDonnell GE, et al. (2012) Aldehyde-resistant mycobacteria bacteria associated with the use of endoscope reprocessing systems. Am J Infect Control 40: 880-882.
- Svetlikova Z, Skovierova H, Niederweis M, Gaillard JL, McDonnell G, et al. (2009) Role of porins in the susceptibility of Mycobacterium smegmatis and Mycobacterium chelonae to aldehyde-based disinfectants and drugs. Antimicrob Agents Chemother 53: 4015-4018.
- Yang FC, Yan JJ, Hung KH, Wu JJ (2011) Characterization of ertapenem-resistant Enterobacter cloacae in a Taiwanese university hospital. J Clin Microbiol 50: 223-226.
- Srinivasan A (2003) Epidemiology and prevention of infections related to endoscopy. Curr Infect Dis Rep 5: 467-472.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences