To Assess the Efficacy Outcomes of Patients Undergoing Androgen Deprivation Therapy in a Tertiary Care Hospital: A Retrospective Study
Eldhose Elias George1, Anjali Ambrose1, Dr. Shaji George2* and Dr. Judith Aaron3
1Department of Pharmacy Practice, Nirmala College of Pharmacy, Muvattupuzha, Kerala, India
2Department of Health Sciences, Holy Grace Academy of Pharmacy, Mala, Thrissur, India
3Department of Radiation Oncology, Caritas Hospital, Thellakam, Kottayam, India
- *Corresponding Author:
- Shaji George
Department of Health Sciences,
Holy Grace Academy of Pharmacy, Mala, Thrissur,
India,
E-mail: shajige@gmail.com
Received date: August 29, 2022, Manuscript No. IPJPM-22-13917; Editor assigned date: August 31, 2022, PreQC No. IPJPM-22-13917 (PQ); Reviewed date: September 12, 2022, QC No IPJPM-22-13917; Revised date: September 22, 2022, Manuscript No. IPJPM-22-13917 (R); Published date: September 29, 2022, DOI: 10.36648/2572-5483.7.9.164
Citation: George EE, Ambrose A, George S, Aaron J (2022) To Assess the Efficacy Outcomes of Patients Undergoing Androgen Deprivation Therapy in a Tertiary Care Hospital: A Retrospective Study. J Prev Med Vol.7 No.9:164
Abstract
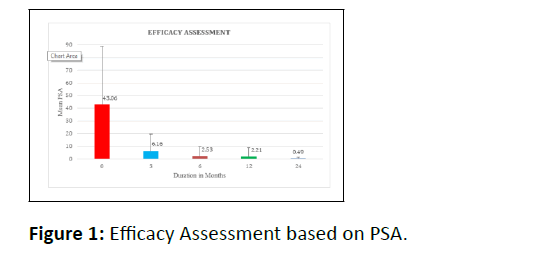
Prostate cancer has become a big concern globally, with over one million cases diagnosed each year and over 300,000 fatalities, making it the sixth highest cause of cancer death in males. Androgen Deprivation Therapy (ADT) is one of the most often used treatments for prostate cancer; thus, determining its safety and efficacy is critical. The study's goal was to evaluate the efficacy of androgen deprivation therapy in prostate cancer patients. From 2013 to 2019, patients having radiation therapy and receiving ADT were studied in this single-center, retrospective, observational study in a tertiary care hospital in Southern Kerala with frequent follow-up for one year. Medical records were used to gain patient data for the analysis and then entered into a data collection form. The study enrolled a total of 140 participants. The results reveal that the mean of the initial PSA (Prostate-Specific Antigen) values obtained was 43.06 and that after three months, the mean value of PSA was 6.16, six months, 2.53, one year, 2.21, and finally, two years, the mean value of PSA was 0.49. The study shows a reduction in PSA values progression in the patients undergoing androgen deprivation therapy. Patients who took ADT had their PSA values decrease in progression, and the benefit of ADT was shown to be best when treated earlier.
Keywords
Prostate cancer; Androgen deprivation therapy; Prostate-specific antigen; Efficacy
Introduction
Anatomy of Prostate
The prostate is a male gland about the size of a walnut placed right under the bladder, in front of the rectum, and surrounds the urethra, a tube that takes urine out of the bladder. The human prostate is a pelvic gland located in front of the rectum, beneath the urine bladder. It is made up of glandular and nonglandular entities contained in a single capsule. It's muscularfibrous tissue and is divided into 50 tubule-alveolar glands near the prostatic urethra's posterior wall, draining to 20-30 tiny prostatic ductless apertures in the prostate. The prostate is around 3 cm in length and 20 grams in weight. Its job is to produce around a third of all seminal fluid. The prostate is primarily glandular tissue, which makes fluid that contributes to roughly 30% to 35% of the semen. This prostatic component of the semen feeds the sperm and provides alkalinity, which aids in maintaining a high pH. The seminal vesicles produce the rest of the seminal fluid [1].
Prostate Cancer
cancer is the most frequent cancer in men worldwide, and it is also the fourth leading cause of cancer death in men. In the United States, prostate cancer is relatively frequent and often begins without symptoms. Prostate cancer will be diagnosed in 164,690 men in 2018, according to the American cancer society, and 29,430 men will die from it. Prostate cancer has become a serious global problem, with an annual diagnosis rate of over one million cases and a mortality rate of over 300,000 deaths per year, making it the sixth-largest cause of cancer death in men globally [2]. It is the second most common cancer in men worldwide, according to GLOBOCAN 2018, and the fifth-greatest cause of cancer death. Prostate cancer affects one out of every nine men at some point. Because prostate cancer is slow-growing cancer, many men die from conditions other than cancer. However, most prostate cancers are aggressive and can migrate outside of the prostate gland, posing a severe risk of death. Prostate cancer survival rates can be improved with early identification and customized treatment [3].
Androgen Deprivation Therapy
Androgen deprivation therapy is one of the most used therapies for advanced prostate cancer. ADT benefits as a treatment option or in combination with other therapies such as prostatectomy or radiotherapy in men with locally advanced prostate cancer have been established. Prostate cancer is caused by androgen receptor transcription factor stimulation by androgen steroid hormones [4]. Androgens are also required for prostate cancer to thrive. Androgens promote the growth of both standard and cancerous prostate cells by binding to and activating the androgen receptor, a protein found in prostate cells. Once activated, the androgen receptor causes the production of specific genes that cause prostate cells to proliferate. Almost all testosterone is produced by the testicles, with only a small amount paid by the adrenal glands. Although prostate cancer cells are generally not capable of producing testosterone, even though some do produce [5].
Indication for ADT
The most prevalent candidates for ADT include men with intermediate- to high-risk localized prostate cancer undergoing radiation therapy, biochemical recurrence following radical prostatectomy treated with salvage radiation therapy, or metastatic prostate cancer. ADT can be accomplished in several methods, including lowering testicular androgen secretion, limiting circulatory androgen activity at the receptor level, using anti-androgens, and combining suppression and inhibition to produce complete (or maximal or total) androgen blockade [6].
Relevance of the Study
Prostate cancer has become a rising problem worldwide, with annual diagnoses exceeding one million cases and a mortality burden of over 300,000 fatalities. According to Globocan 2020, 34,540 new instances of prostate cancer were reported in India, placing it 12th among all cancers. Because there have been no studies on the efficacy of ADT in the treatment of prostate cancer in a tertiary clinical care environment in Kerala in recent years, the necessity for one arises in this context. When androgen deprivation therapy is required, the study will be able to provide precise therapeutic recommendations to clinicians in selecting the best medicine, which will be beneficial in personalizing the drug regimen.
Applications/Socio-Economic Importance
Establishing the benefits of ADT in prostate cancer patients is of social importance. Provide relevant information on the appropriate use of ADT in treating patients with prostate cancer. Because ADT can shrink tumors, it's only necessary to utilize lesser doses of radiation therapy. As a result, many of the radiation therapy's cumulative adverse effects can be mitigated. The same holds when administered as an adjuvant to radiation. When a patient refuses surgery for whatever reason, including financial restraints, we can utilize ADT in conjunction with monoclonal antibodies as an effective therapy. Further advancements in ADT, such as androgen antagonists, may lower the cost of many radiation treatments and the chance of developing unrelated cancers. The goal of the trial was to see how effective androgen restriction therapy was in patients with prostate cancer.
Materials and Methods
Study Design
From 2013 to 2019, a retrospective, observational singlecentre study was conducted on prostate cancer patients who had had radiation therapy and were undergoing ADT at CARITAS Hospital, with frequent follow-up for one year to determine the effect of Adjuvant Androgen Deprivation Therapy. On the 5th of September, 2020, the hospital's Ethical Committee approved. The study followed the Declaration of Helsinki's ethical guidelines and additional procedures such as the Good Clinical Practice Guidelines and those developed by the ICMR.
Patient Selection
Cochran’s formula for calculating sample size was used in the population with a definite number:
n0=Z2Pq/e2
where,
n0 is the sample size,
Z is the selected critical value of desired confidence, level,
P is the estimated proportion of an attribute that is present in the population
q=1-p
e is the desired level of precision
n= n0/(1+ (n0 - 1)/N)
Here,
n0 is the sample size derived from the equation
N is the population size
Selection of Study Population
Patients were selected randomly on the basis of inclusion and exclusion criteria.
Inclusion criteria
1. Age above 18 yrs.
2. Gleason score & gt; 3
3. PSA & gt; 20
4. Biopsy +ve, Rectal examination +ve
5. Undergone Radiotherapy
6. On ADT between 2013-2019
7. Tolerating therapy and on regular follow up for a minimum of one year
8. Prostate cancer patients willing to participate
9. Patients having a life expectancy of more than 12 months
Exclusion criteria
1. Patients on palliative care for prostate cancer
2. Patients having other types of cancer
3. Patients undergoing other treatment regimens.
4. Patients having brain metastasis
5. Patients with a history of seizures
6. Patients using herbal products having anticancer activity
Data Collection
Because there were no computerized prescribing records, relevant information about the patient was manually gathered from the patient's case file, which was available at the Nurses' Station. After that, the information was entered into the data gathering form. The patient's age, height, weight, BMI, Gleason score, PSA levels, testosterone values, medicines and dose provided, and Adverse Drug Reactions (ADR) observed throughout the therapy period were gathered retrospectively.
Result
Statistical Analysis
Assessment of efficacy (Table 1,2, Figure 1)
| PSA (Duration in Months) | N | Mean | Std. Deviation |
|---|---|---|---|
| 0/ Initial PSA | 140 | 43.06 | 45.63 |
| 3 | 137 | 6.16 | 13.44 |
| 6 | 122 | 2.53 | 7.01 |
| 12 | 107 | 2.21 | 6.65 |
| 24 | 61 | 0.49 | 1 |
Table 1: Mean values of PSA over different time periods with standard deviations.
| Pair | Mean | Std. Deviation | 95% CI | t | df | P value | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Initial PSA-3 month | 37.46 | 44.92 | 29.87 | 45.05 | 9.76 | 136 | 0 |
| 3 month-6month | 4.13 | 12.66 | 1.86 | 6.4 | 3.61 | 121 | 0 |
| 6 month-1yr | 0.6 | 5.8 | -0.53 | 1.73 | 1.05 | 102 | 0.3 |
| 1yr-2yr | 2.73 | 7.9 | 0.71 | 4.76 | 2.7 | 60 | 0.01 |
Table 2: Paired sample T-test of Assessment of Efficacy.
Paired Sample T-test
Hypothesis
Ho: There is no significant difference between the PSA values initial 3 months, 3&6 months, 6&1year, 1year, and 2year.
H1: Ho is false.
Discussion
The p values for the pairs Initial PSA-3 months, 3month-6 months, and 1 to 2 years are all 0.000.05, indicating a significant variation in the PSA readings. However, the value of 0.30 in pairs (6 months-1 year) is more than 0.05, showing no significant difference in the PSA values. Patients with clinically localized or locally progressed prostate cancer can attain desired PSA levels with androgen deprivation therapy. It can maintain low PSA levels with continued treatment, according to the findings of this study. The patients treated with Androgen Deprivation Therapy showed a decline in the values of PSA. The PSA values obtained after the treatment with ADT showed a significant decline. Similarly Gleason Score of the patients were also significant after the treatment with androgen deprivation therapy. Gleason score was measured after the completion of five ADT treatments. The testosterone values of the patients undergone ADT was measured after five successive therapies and was found be to significant, suggestive the benefits of ADT. When individuals are treated sooner, the therapeutic benefits of androgen deprivation therapy are most significant.
Short-term ADT was connected to a lower disease-specific mortality rate and a greater overall survival rate in a study published (2011). According to the study, short-term ADT did not enhance overall survival or reduce disease-specific mortality in men with low-risk diseases after ten years. However, it dramatically reduced biochemical failure and positive findings on repeat prostate biopsy after two years [7]. This reveals that if hormone-blocking is done early in the treatment of low-tumourburden males, they can expect to live longer, supporting the rationale for its use in limited disease. According to a study, combination hormone-blocking is particularly successful in controlling clinically localised or locally advanced prostate cancer (2002) [8]. Despite the lack of randomised clinical trials demonstrating a long-lasting or widely reported clinical impact on castration resistance, bicalutamide 50 mg per day is commonly used in practice for men who have failed ADT. In the United States, bicalutamide 50 mg per day was approved based on a trial of males with hormone-naive metastatic disease who were given flutamide as a comparison; both were given in combination with luteinizing hormone-releasing hormone analog treatment [9]. In the absence of approved therapies, the use of bicalutamide shifted to the treatment of castrationresistant diseases.
The information gathered determined that the treatment effect of androgen deprivation therapy medications on the patients in question was consistently positive. As a result, androgen restriction therapy has been linked to a lower incidence of prostate-specific antigen advancement.
We could not attain an appropriate sample size for our investigation due to unforeseen COVID-19 pandemic circumstances, which was an unavoidable limitation throughout our research. Even though the number of patients having ADT in our facility was sufficient to undertake a study, many were excluded. More patients may be included if this was a multicenter study rather than single centre research. The patient chart lacked certain information. Difficulties arose in the study as a result of missing follow-up information.
Summary
The study looked at the safety and efficacy of androgen deprivation therapy in patients with prostate cancer. Around 140 patients who had ADT in a tertiary hospital between 2013 and 2019 had their data collected, organised, and analysed using a data collecting form. Leuprolide and Bicalutamide were the most common medicines used in ADT. The safety of the medication was determined by side effects, while the efficacy was determined by PSA and Testosterone levels gathered during the treatment period. Body ache accounted for 21% of all side symptoms, followed by constipation (13%), dysuria (12%), gastritis (11%), nocturia (10%), and painful micturition (10%).
Conclusion
The study's primary goal was to evaluate the efficacy of ADT in PC patients. Patients who took ADT had their PSA values decrease in progression, and the benefit of ADT was shown to be best when treated earlier.
Abbreviations
ADR: Adverse Drug Reaction
BMI: Body Mass Index
CAB: combination androgen blockade
Gt.: Greater
PC: Prostate Cancer
PCa: Prostate Cancer
PSA: Prostate specific antigen
Declarations
Ethical Approval and Consent to participate
The scientific review board has reviewed our proposal and has approved the referenced project to be conducted at their hospital under the supervision of Dr. Shaji George and Dr. Judith Aaron.
1. Consent for publication: Not Applicable
2. Availability of data and materials: Data and materials are personally available with research authors
3. Competing interests: The authors declare that they have no competing interests
4.Disclosure Of Funding: None
5. Authors' contributions
The first author (Eldhose Elias George) drafted the manuscript, the second author (Anjali Ambrose) collected the literature and references for the manuscript and together edited the manuscript and, the corresponding author (Dr. Shaji George) supervised the entire progress and reviewed the manuscript and the Fourth author (Dr. Judith Aaron) contributed to the visualization, investigation, and validation.
6. Acknowledgment
The author would like to thank the staff and the postgraduate students of the Department of Pharmacy Practice, Nirmala College of pharmacy, Muvattupuzha and Dr. Shaji George for his support and encouragement, and Dr. Judith Aaron for her valuable guidance and validation.
References
- Toivanen R, Shen MM (2017) Prostate organogenesis: Tissue induction, hormonal regulation and cell type specification. Development 144: 1382-1398.
[Crossref], [Google Scholar], [Indexed]
- Litwin MS, Tan HJ (2017) The Diagnosis and Treatment of Prostate Cancer: A Review. JAMA 317: 2532-2542.
[Crossref], [Google Scholar], [Indexed]
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA et al. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68: 394-424. [Crossref], [Google Scholar], [Indexed]
- Scher HI, Sawyers CL (2005) Biology of progressive, castration-resistant prostate cancer: Directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol 23: 8253-8261.
[Crossref], [Google Scholar], [Indexed]
- Dillard PR, Lin MF, Khan SA (2008) Androgen-independent prostate cancer cells acquire the complete steroidogenic potential of synthesizing testosterone from cholesterol. Mol Cell Endocrinol 295: 115-120.
[Crossref], [Google Scholar], [Indexed]
- https://www.cancer.org.au/
- Jones CU, Hunt D, Mc Gowan DG, Amin MA, Chetner MP et al. (2011) Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med 365: 107-118.
[Crossref], [Google Scholar], [Indexed]
- Labrie F, Candas B, Gomez JL, Cusan L (2002) Can combined androgen blockade provide long-term control or possible cure of localized prostate cancer? Urology 60: 115-119.
[Crossref], [Google Scholar], [Indexed]
- Schellhammer PF, Sharifi R, Block NL, Soloway MS, Venner PM et al. (1996) A controlled trial of bicalutamide versus flutamide, each in combination with luteinizing hormone-releasing hormone analogue therapy, in patients with advanced prostate carcinoma. Analysis of time to progression. CASODEX Combination Study Group. Cancer 78: 2164-2169.
[Crossref], [Google Scholar], [Indexed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences