The True Nature of CurcuminÃÆâÃâââ¬Ãâââ¢s Polypharmacology
Franco Cavaleri and William Jia
DOI10.21767/2572-5483.100015
Franco Cavaleri1* and William Jia2
1Faculty of Medicine, Department of Experimental Medicine, Center for Brain Health UBC Hospital, Canada
2Division of Neurosurgery, Department of Surgery, Center for Brain Health UBC Hospital, Canada
- *Corresponding Author:
- Franco Cavaleri
Faculty of Medicine, Department of Experimental Medicine
Center for Brain Health UBC Hospital, Canada
Tel: 1855-518-8858
E-mail: franco.c@biologic-med.com
Received date: September 18, 2017; Accepted date: October 26, 2017; Published date: November 2, 2017
Citation: Cavaleri F, Jia W (2017) The True Nature of Curcumin’s Polypharmacology. J Prev Med Vol.2 No.2:2 doi:10.21767/2572-5483.100015
Copyright: © 2017 Cavaleri F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Curcumin, a concentrated extract of the herb, turmeric, is a widely used medicinal agent purported to deliver significant anti-inflammatory and other pharmacological activities. As much as curcumin presents a narrower range of pharmacological activity over the whole herb, the extract is still comprised of multiple curcuminoid constituents that contribute to a polypharmacology. Curcumin has been administered successfully in clinical trials to treat depression; Alzheimer's and other neurological diseases; autoimmune and auto inflammatory disorders; and various types and stages of cancer with reasonable success but also with conflicting reports. Upon meta-analysis of the plethora of curcumin-related research it is evident that the pharmacology of this extract is not fully understood.
The naturally occurring, highly homologous curcuminoid analogues are assumed to partake in similar and additive pharmacological events due to their common structural features. However, a perspective shift is presented to feature their electrochemical and structural differences and highlight the potential for each curcuminoid to also exhibit unique pharmacology that is distinct from the others. This, in part, is shown to help explain curcumin's polypharmcology when administered to treat multiple diseases that manifest with pathological features and symptoms, nevertheless, that are quite diverse.
A full review explains how highly hydrophobic and extremely reactive curcuminoid chemistry can still be administered orally; is bioavailable despite conflicting reports that it is not; and delivers efficacious pharmacology systemically as a direct function of the parent curcuminoid molecules and in an additive manner through the accompanying auto-oxidative degradation by-products of the parent curcuminoids.
Keywords
Anti-inflammatory; Curcumin; Polypharmacology; Curcuminoid; Turmeric; Cancer; Alzheimer's disease
Introduction
Curcumin (diferuloylmethane) is a major active constituent of turmeric (Curcuma longa) [1] with an expansive pharmacology including anti-inflammatory [1], anti-carcinogenic [2], wound healing [3] and antibacterial [4] to name just a few features. Its safety is well established by centuries of use in food and traditional medicine [5-7]. Subcellular signalling proteins such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) [8], c-Jun N-terminal kinase (JNK) [9], Protein Kinase C (PKC) [10], AKT and mechanistic target of rapamycin (mTOR) signalling [11,12], and mitogen-activated protein kinases (MAPKs) [13] have made a sizeable list of curcumin’s pharmacological targets that continues to evolve. Additionally, these targets are central to the pathology of diseases that are prolific in society today such as neurological disease [14,15], autoimmunity [16,17], cardiovascular disease [18,19] and even cancer [20]. This all makes for a rather exciting story for curcumin as a potential medicinal agent.
The very fact that the list of targets and mechanisms of activity by curcumin continues to grow is, itself, demonstrative of our incomplete understanding of the fundamental underlying mechanism by which curcumin pharmacology modulates disease pathology. Studies have shown curcumin to inhibit growth factors and growth factor receptors as well as the downstream signals including PI3K and extracellular-signal activated kinase (Erk); and oncogenes such as c-jun and c-myc [21,22]. The extract is shown to inhibit expression of epidermal growth factor receptor (EGFR) and erythroblastosis oncogene B (ErbB2) [23]; inhibit enzymes such as cyclooxygenase (COX) and lipoxygenase (LOX)[24-27]; facilitate transcription factors such as nuclear factor erythroid 2-related factor (Nrf2) [28] that can contribute to endogenous antioxidant status and protect cells from oxidation; while it inhibits activator protein – 1 (AP-1) and tumor necrosis factor α (TNFα)[29,30]. Curcumin is shown to inhibit cytokines such as interleukins 1, 2, 6 and 8 [25,30-32]. The extract is also shown to suppress Interleukin (IL)-12 in macrophages [33] while promoting the anti-inflammatory IL-10 [34]. How is all this possible and how can this be harnessed and controlled?
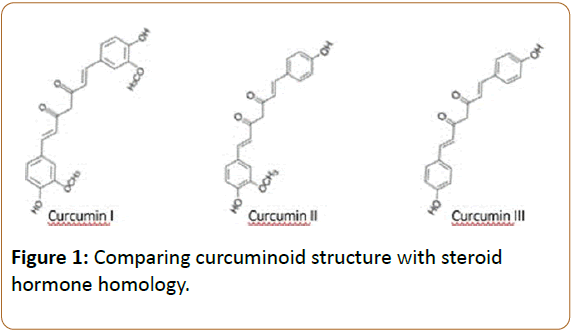
Curcumin comprises a subset of active constituents. The three main naturally occurring curcuminoid analogues found within the curcumin extract are diferuloylmethane (curcumin I), desmethoxycurcumin (curcumin II) and bis-desmethoxycurcumin (curcumin III) [35,36] as seen in Figure 1. They typically exist naturally in proportions that range between 65-80% curcumin I, 10-25% curcumin II and 0.2-3.0% curcumin III [37]. Curcumin delivers a polypharmacology that exhibits a narrower range of activity than the whole herb [38,39]. However, the curcumin extract is certainly not maximally selective as it is still delivering multiple active constituents each potentially exhibiting a polypharmacology of their own.
There is yet another component of the pharmacology that may be contributing to curcumin’s polypharmacological nature. The sub-constituent curcuminoids readily give rise to autooxidative degradation products [40-42], some of which we know to exhibit pharmacological activity that we may have attributed to the curcuminoids in the past. Despite the conflicting findings on these curcuminoid by-products, they may be playing a monumental role in the pharmacology of this remarkable extract; a role that needs to be viewed with a more focussed lens.
Clinical Benefits of Curcumin
Since long ago, oral dosing of curcumin with as little as 20 mg three times daily has been shown to improve acute and chronic hepatitis [43]. Curcumin is a potent cholegogue inducing gallbladder contraction and bile elimination [44] conducive to bladder stone management. However, the more recent understanding of curcumin’s anti-inflammatory pharmacology and what this means in the context of disease management has elevated interest in the extract as a potential treatment for many modern epidemics. Curcumin is also shown clinically to enhance cytotoxicity of various drug-resistant strains of cancer [45].
In clinical trials patients with various cancer-related risks including bladder cancer, cervical cancer, intestinal metaplasia and oral leukoplakia were treated with systematically escalating doses up to 8000 mg daily of curcumin for three months [46]. Results were indicative of a significant anti-cancer effect by the curcumin treatment with relatively little to no toxicity. In murine models curcumin is also shown to ameliorate functional and structural abnormalities associated with cancer drug cisplatininduced neuropathy [47].
In the treatment of orbital pseudotumors curcumin produces significant positive results. After following these patients for as long as two years at three month intervals, four patients recovered completely among five who stayed in the study to completion. One patient experienced complete regression but with some limited movement as a residual symptom [48]. The treatment of psoriasis by oral curcumin administration is shown to produce an excellent therapeutic outcome in two patients encouraging the need for larger controlled trials [49]. Topical application of curcumin preparation shows curcumin treatment to produce a more profound resolution than calcipotriol or nontreated (control) patients with various degrees of psoriasis [50].
A twenty-four week double blind placebo-controlled study resulted in an inconclusive position on curcumin’s effects on Alzheimer’s patients [51]. Curcumin use did show signs of β- Amyloid changes in serum indicative of β-Amyloid disaggregation and a tendency towards fewer adverse events for patients using curcumin. However no improvement in cognitive performance in the curcumin group over controls is established. However, since the control group did not shown cognitive decline during the period of the trial, the study design will need to be modified to be able to better evaluate these outcomes; better controls, larger groups and longer trials are expected requirements acknowledged by the authors.
However, researchers have not given up on the extract when it comes to amyloidogenic diseases. In vivo murine studies show that curcumin does cross the blood brain barrier [52,53] and binds to amyloid plaques when orally fed or directly injected into the carotid artery [54]. When coupled to the known in vitro results associated with Alzheimer’s disease biomarkers these findings are suggestive of efficacy against Alzheimer’s pathology [55]. Other studies show curcumin improves cognitive function in patients with Alzheimer’s disease [53]. More research is required to further define curcumin’s clinical efficacy and mechanisms involved in the framework of Alzheimer’s pathology.
Curcumin is shown to deliver anti-depressant like activity similar to that of fluoxetine and imipramine [56] and the mechanism might involve increasing brain derived neurotrophic factor (BDNF) [57]. On the other hand curcumin’s antidepressant- like activity maybe a function of inhibitory activity on IL-6 and IL-1 [35,58] since dysregulation of these cytokines and the systemic inflammatory activity that can rise from this may contribute to depression pathology [59,60].
Curcumin is shown to inhibit p300-HAT to improve cardiac hypertrophy and heart failure in animal models [61,62]. Curcumin delivers better results than diciofenac sodium in a recent study of patients with active rheumatoid arthritis comparing these therapies [6,63]. Curcumin appears to correct cystic fibrosis transmembrane conductance (CTFR) defects associated with cystic fibrosis (CF) in murine models [64]. Curcumin administration stimulates muscle regeneration after traumatic injury [65]. Curcumin improves COPD-like airway inflammation [66].
All those in a small group of five ulcerative colitis patients improved using 550 mg curcumin twice daily as treatment for a month followed by a month of three daily 550 mg doses [7].
Curcumin pharmacology looks promising to say the least but these remarkable results are also in conflict with similar studies showing lack of success in clinical and preclinical models with curcumin treatment [67] such as in depression models where curcumin’s effects are found to not be significant [68]; where in other independent studies of CTFR (CF) defects curcumin’s benefits were not repeatable [69]; and in research using high curcumin doses to treat inflammatory conditions such as rheumatoid arthritis patients experienced improvements as much as but not more than those receiving phenylbutazone [7].
Curcumin pharmacokinetics
Curcumin bioavailability
Despite the abundance of experimental and anecdotal clinical evidence demonstrating the health benefits of orally routed curcumin, limited to no serum curcumin is found in test subjects even at extremely high dosing that exceeds 10,000 mg daily [70,71]. The reasons for the low tissue or serum availability appear to be due to multiple compounding factors including low bioavailability [72] and expeditious metabolic degradation [73] that causes rapid elimination of the curcuminoids [72]. The naturally occurring curcuminoid analogues are highly hydrophobic [74,75], a characteristic thought to play a major role in bioavailability [72]. Overcoming the hydrophobic characteristics of the curcuminoids resolves only one of the challenges, however. There are other curcuminoid issues related to pharmacokinetics that are outstanding and are likely far more central to the understanding and efficacy of curcuminoid pharmacology than the bioavailability limitation.
The low bioavailability of curcumin is assumed due to the lack of serum curcuminoids [70] and the common excessive efflux of some curcumin preparations in fecal matter upon oral administration [76]. In comparison with intraperitoneal administration of pure curcumin extract which excludes the tumerone fraction, 75% of orally administered curcumin extract was excreted in feces with more than 10% found in bile [76] in a mouse model. In human patients, Cheng et al report that even with 8000 mg of oral curcumin administered daily, serum concentrations were found to be 1.77 +/- 1.87 microM [46]. In colorectal patients taking up to 3600 mg of curcumin orally daily neither curcumin nor its metabolites were found at quantifiable levels in plasma, blood and urine [77]. In a human Phase I clinical trial, Sharma et al found curcumin and its metabolites in plasma in the 10 nM range after oral dosing as high as 3600 mg daily [70]. In the treatment of pancreatic cancer using orally administered curcumin plasma curcumin levels are found to range between 22-41 ng/ml [71].
Poor curcumin/curcuminoid bioavailability is thought to be caused by the highly hydrophobic property of the phenolic compounds [75]. Many strategies have been applied to overcome the hydrophobicity of curcuminoids in an attempt to improve bioavailability such as interacting them with beta-casein (micellar casein) to improve solubility in aqueous mediums [78]; encapsulation of curcuminoids in hydrophobically modified starch [79], and phosphatidylcholine interactions with curcuminoids to enhance bioavailability and delivery [80,81]. Administration of complexed curcuminoid-phosphatidylcholine is in fact shown to deliver a higher serum payload of curcumin over curcumin powder alone [82]. However, more detailed studies might be needed to determine if the incremental serum curcuminoids found with this reacted curcuminoid complex is a function of improved solubility and bioavailability. Could the improved survival of serum curcuminoids be a function of recipient-induced alteration in hepatic enzyme activity that may reduce the clearance rate of curcuminoids from blood? In addition, feed type [83], fiber content [84] and many other factors can also play into gastric emptying rate [85] gastrointestinal transition rate and macronutrient digestion and absorption [86]. This all influences drug transition rate and bioavailability as well and are not always fully accounted for in these studies.
The bioavailability limitation of curcumin, however, may be also overstated because studies also show that curcumin can efficiently find its way into serum at concentrations that are rather significant [51]. Thirty-four subjects of a six-month trial using powdered curcumin versus encapsulated curcumin presents a different bioavailability story. This study shows a mean plasma curcumin level of 490 nM amongst both curcumin groups but an interestingly higher (940 nM) level for the curcumin capsule group over the group fed curcumin powder at the daily dose of 1.0 gram daily. A group using 4.0 grams daily was also evaluated but serum curcumin results with this higher dose was not significantly higher.
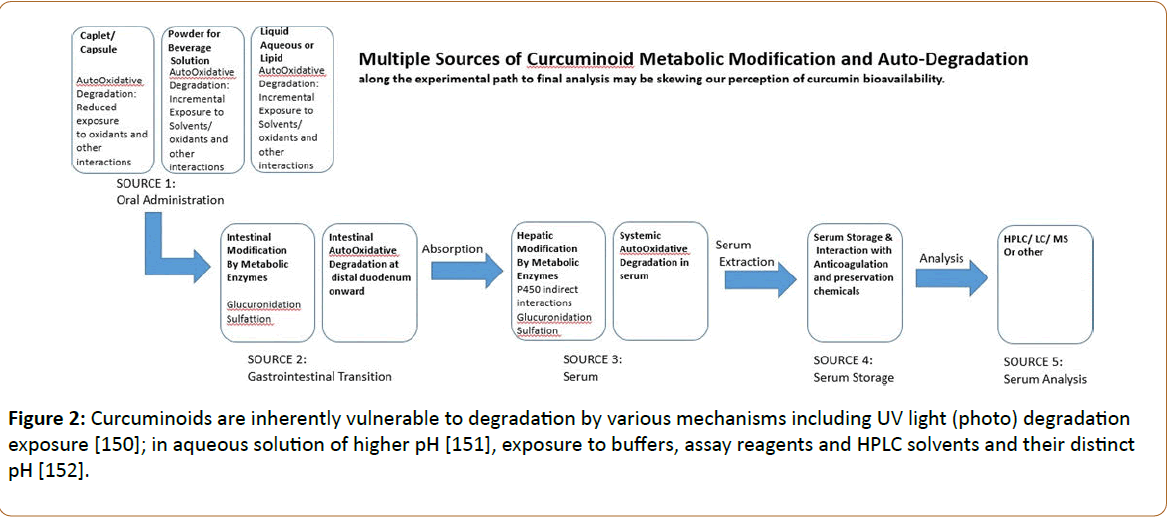
However, it’s interesting to note that while curcumin levels differed, levels of tetrahydrocurcumin, ferulic acid and vanillic acid did not differ between patients using powdered curcumin and those using capsules. The powder form could be performing less effectively due to the need to mix it in aqueous or other solutions that allow the auto-degradation process to start in on curcuminoid degradation long before it even enters the lumen. Interestingly, serum levels of curcumin could only be detected in the presence of glucuronidase inhibitor [51]. Here we have a clear indication that bioavailability of curcumin can be functional and maybe, the serum limitations are more attributable to shortfalls in curcumin formula design and post absorption modification and degradation that play a larger role in serum survival. Figure 2 presents a schematic that highlights multiple sources of curcumin/oid degradation that could affect curcumin “apparent” bioavailability. This degradation starts with the type of curcumin delivery form or formula and carries through to the final reagents and solvents used in analysis.
Curcumin metabolism
It is well understood that metabolic degradation of curcumin is rapid and efficient. In the preliminary study by Baum et al [51] it was determined that serum curcumin could be increased within 1.5 hours of oral administration with food to 250 nM and to 270 nM by four hours with water only. By twenty-four hours post-administration serum curcumin levels fell to 60 nM. No significant differences were found between the groups taking 1.0 gram curcumin daily versus the group taking 4.0 grams daily.
This study proves to be one that highlights the true potential of curcuminoid bioavailability; it can be viable for a properly formulated curcumin treatment. Degradation and metabolic modification, on the other hand, may be the more difficult challenge. In fact, in this same study, at 2.5 hours after oral curcumin administration serum ferulic acid is found to be 110 +/- 20 nM, vanillic acid is 50+/-20 nM, total curcuminoids found to be 1100+/-260 nM, tetrahydro curcumin is found to be 440+/-100 nM, and no vanillin is found.
Enzymatic metabolism of curcuminoids starts in the intestinal lumen and is quickly followed by hepatic enzyme activity [87, 88]. Although it is not clear whether Phase I metabolic enzymes such as the P450 CYPs are directly involved in curcuminoid metabolism, their influence may be indirect as explained further here. Once in the blood, for example, curcuminoid survival is prolonged or protected by serum albumin [89] which likely forms micellar systems with the systemic curcuminoid. Cationic micelles of curcuminoid [90], for instance, which can be achieved with beta casein are not only said to improve bioavailability but also protect the curcuminoids from premature degradation [78]. Binding of curcuminoids in vitro to bovine serum albumin (BSA), likely in the protein’s hydrophobic pockets, results in a curcumin-BSA complex with improved curcumin stability [91]. In fact, curcumin solubility is increased as much as 10-fold in the presence of BSA [92].
In vivo, curcuminoids are quickly converted to dihydrocurcumin, tetrahydrocurcumin, hexahydrocurcumin, and hexahydrocurcuminol. These metabolites are quickly further subjected to glucuronation and sulfation to form curcumin glucuronide, curcumin sulfate, dihdrocurcumin glucuroside, tetrahydrocurcumin glucuronoside, and hexahydrocurcumin glucuronoside [73,87,93,94].
This likely involves Phase II metabolic enzymes - UDPglucuronosyltransferases (UGT) and Sulfotransferase enzymes (SULT) [95]. However, the literature is not black and white in this context. To throw another curve in the context of metabolic degradation it must be considered that curcumin metabolic degradation and elimination is shown in some studies to play out differently in the human versus rat model.
The human intestinal and hepatic cytosol is more likely to conjugate curcumin and produce the tetrahydrocurcumin metabolite in place of curcumin more abundantly than the rat model does [96]. What this means to total pharmacological potency is unexplored to date but what has been a problem up until now is the direct extrapolation from murine models to human models with lack of scientific support. More work needs to focus on unravelling this mystery.
There is no strong evidence to show that curcumin is subject to metabolism by P450. However, the Phase I P450 and the Phase II enzymes tend to aggregate at the membrane and influence each other. P450 (CYP), for instance, interacts intimately with UDP-glucuronosyltransferases (UGT) responsible for glucuronidation to form heteromers at the plasma membrane [97] that result in their competition for substrates and down- and up-regulation of activity [97]. It is possible that any substance or influence that affects P450 activity, such as changes in membrane phospholipid constitution, may indirectly influence curcumin’s metabolism [98]. Phosphatidylcholine in curcumin complexes that has been shown to improve solubility and bioavailability of curcumin [81] may also affect P450’s since membrane phosphatidylcholine is thought to be the anchoring phospholipid for at least some P450 enzymes such as 2B4 [99].
Failure to detect functional levels of curcumin in the plasma after a steady oral loading period or administration by other route may also be attributed to instability and non-enzymatic degradation of the curcuminoids. Various human and rat studies demonstrate a short half-life for the curcuminoids [100,101]. Researches have shown that curcumin is more stable in solutions at pH<7.0 while it tends to be less stable in physiological pH of 7.8 or more [102] characteristic of the distal small intestine.
The curcumin non-enzymatic degradation products frequently reported are ferulic aldehyde, trans-6-(40-hydroxy-30- methoxyphenyl)-2, 4-dioxo-5-hexenal, feruloyl methane, ferulic acid and vanillin [102]. Ferulic acid and vanillin, are considered very small phenolic molecules with molecular weights of 151.15 g/mole [103] and 66.8 g/mole [104] respectively. They are soluble in aqueous solution and far more stable than the curcuminoids, themselves, in the biological medium [105-107].
The status of these non-enzymatic auto-oxidative degradation products is also in question and in conflict in the literature as the ones expected to be the major products in the past, vanillin and ferulic acid [42], are said to more recently be preceded by a bicyclopentadione product or other by-products that may not have yet been precisely identified [102,105,108,109]. Too much conflicting data has been presented in this context and although the multiple view-points are great to see for meta-analysis it must be considered that these conflicting positions on the status of the degradation by-products could also be a function of the variable conditions being used to study the curcuminoids.
Variable pH, temperature, serum protein and other conditions that, if even mildly varied, result in varying the stability, degradation dynamic and by-product yield.
These variables could factor into the equation at multiple levels when it comes to in vitro work; but even with in vivo work the feed types, curcumin specifications and animal condition all play a changeable role in the outcome.
Analysis of the blood work extracted including reagents used to treat final yields also influence the stability of the retained target curcuminoids; biochemicals we now know to be extremely vulnerable to degradation. All of these factors as shown in Figure 2 contribute to the variable results and inconsistencies we see in the literature. Somewhere in all this conflict, however, treatment with the right curcumin therapy successfully delivers relief to patients of many diseases.
Ultimately, we need to pin down the pharmacokinetics and isolated pharmacology of the curcuminoids and their downstream by-products in order to eliminate inconsistencies and produce a reliable curcumin-treatment.
Curcuminoids or their Degradation Product?
The enzymatic metabolism of curcumin is shown in some studies to reduce curcumin’s pharmacological potency significantly [110,111]. However, other studies indicate that at least one of these metabolic by-products could be contributing to curcumin’s polypharmacology in tissues [112]. Tetrahydrocurcumin is purported to deliver a significant antiinflammatory pharmacology [110-113]. In fact, more recent studies are pointing to this reduced derivative of curcumin having antioxidant activity and antihyperlipidemic effects at least as potent as curcumin [114]. Tetrahydrocurcumin is shown in some studies to perform well as an inhibitor of NF-kappa-B and protector of oxidative damage after ischemic episodes [115]. While it is shown to deliver more anti-inflammatory activity than curcumin in a carrageenan-induced murine inflammatory model [116] it performs not nearly as well as curcumin in other studies [117]. Other studies again, show varied activity with curcumin performing better than tetrahydrocurcumin on targets like COX-2 inhibition [118].
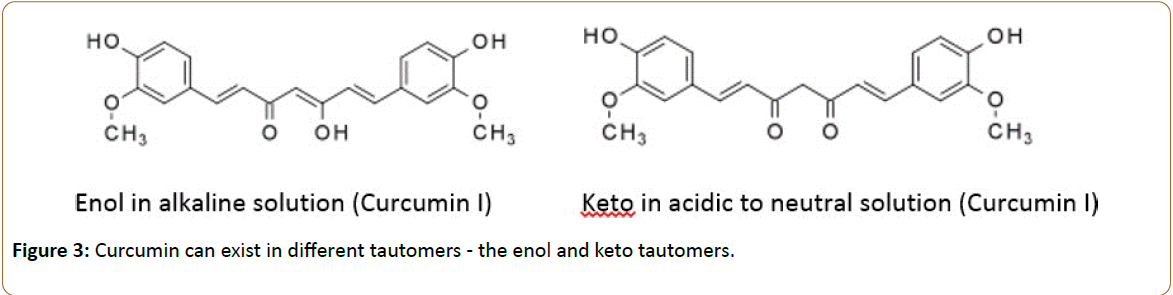
Aside from metabolic degradation as a factor altering curcuminoid pharmacology, the curcuminoids can exist as different tautomers - the enol and keto tautomers, [119,120] as shown in Figure 3. The keto form predominates in a solution of pH 3-7 while at a pH above 7.8 the enol form predominates in solution [121]. The enol form (>pH 7.8) serves as an electron donor while the keto form (pH 3-7) serves as a hydrogen atom donor; although both forms can serve as antioxidant. Nevertheless, the environment in which the curcuminoid exists influences its electrochemical properties factoring, yet again, as another source of pharmacological variability.
The story with regards to the non-enzymatic auto-degradation products of curcumin is even more colourful and adds even more mystery. Curcuminoids also undergo non-enzymatic autooxidative degradation and as we’ve seen this is more likely to occur at pH>7.0 [102]. It was shown that as much as 90% of curcuminoids are degraded within 30 minutes in a serum-free medium at pH 7 at 37°C [122]. Even in the presence of serum, 50% of curcumin is degraded to its degradation by-products within eight hours [102]. The true nature of this degradation yield is still not conclusively understood.
Research by Martelli et al show that curcumin activates the transient receptor potential cation channel subfamily V member 1 (TRPV1) also known as the vanilliod receptor 1. This receptor is the target of vanillin, a degradation product of curcumin. By this mechanism vanillin and/or curcumin could be inducing symptomatic relief of Dinitrobenzene sulfonic acid (DNBS) - induced colitis in mice [123]. Multiple studies point to NF-kappa- B inhibition by curcumin and this being the root activity that results in subsequent IL-1, IL-6 and IL-8 inhibition [124-126].
Similarly, vanillin can also inhibit NF-kappa-B and caspase-1 [127]. COX is inhibited by vanillin [127]. In fact, vanillin’s effects are COX-2 specific delivering the beneficial pharmacology associated with nonsteroidal anti-inflammatory drugs. Interestingly, curcumin is shown to inhibit COX as well [128,129]. Kim et al also show that vanillin protects rat neurons from oxidative stress [130]. Curcumin does the same by inducing expression of antioxidant defensive genes through Nrf2 activation [28,131].
Ferulic acid, another curcumin degradation product, displays pharmacological activity similar to curcumin’s as well. Studies demonstrate that ferulic acid supplementation can facilitate hypotension through NO-mediated vasodilation [132]; a result also seen with curcumin administration [133]. Ferulic acid is shown to have significant antitumor activity [134] as does curcumin [135]. Ferulic acid is shown to inhibit NF-kappa -B [136]. Curcumin has been shown to destabilize preformed β- amyloid protein including inhibition of soluble oligomer and fibril aggregation to subsequently or also independently reduce associated neurotoxicity by these proteins [54,137-141]. Ferulic acid is shown to have similar activities in vitro [130,139,142,143].
Similar pharmacological activities of curcumin and its oxidative degradation products strongly suggest a contribution by curcumin’s auto-oxidative degradation products to curcumin pharmacology in vivo. This may explain the therapeutic results with curcumin administration despite low bioavailability or more accurately low serum levels of the curcuminoid analogues. The fact that we experience efficacious results with oral curcumin administration with or without the identification of significant serum curcuminoid concentrations supports the notion that vanillin, ferulic acid and/or other degradation products of curcumin may be responsible at least partially for the clinical benefits of curcuminoids.
The variety of experimental models used to investigate curcumin includes in vitro and in vivo studies using various representations of turmeric and the common extract, curcumin. Curcumin’s pleiotropic properties certainly make it a versatile molecule. The question is whether this pleiotropy is a function of one curcuminoid analogue on multiple targets, the naturally inherent three curcuminoid analogues, the degradation products, or all of these factors? A better understanding of the complex nature of this activity can help us decode and identify the active components contributing to the polypharmacology. With this mapping, improved selectivity by curcumin-based drugs can be established and improved indication-specific drug designs with improved reliability and repeatability can be created. As we have it today, too many variables are at play.
Conclusion
One of the challenges faced today with respect to curcumin acceptance in mainstream medicine is its polypharmacology or lack of clear cellular targeting. Drug target selectivity is central to allopathic drug design for reasons that are valid [144,145]. However, an emerging drug paradigm that centers on polypharmacology [145,146] to produce a synergistic therapeutic outcome is gaining some momentum for reasons that may also be valid. In essence, polypharmacology as a treatment model is already established in mainstream allopathic medicine and is in use to treat many complex disorders today including autoimmune disease [147,148] and especially cancers [146,149]. Interestingly, curcumin falls into this class of drug perfectly; however, in order to better understand the entire scope of this polypharmacology by curcumin much more work needs to be done.
It is evident that curcumin extracts are made up of multiple naturally occurring curcuminoid analogues that must be studied in isolation in order for the distinct pharmacological features for each to be better defined. This may help formulators produce condition-specific products using the curcuminoid analogues with greater precision. In addition, it must be made very clear whether we have an influence in the in vivo model by the autooxidative by- products of curcumin or any of the enzymatic degradation products such as tetrahydrocurcumin. The level of contribution not only as constituents to serum bioactives but more accurately the tissue distribution of these potentially active degradation products must be defined.
Serum curcumin levels appear in the literature to not correlate well with efficacy of curcumin-based treatment protocols. Despite low to no serum curcumin upon oral administration in some studies efficacy against various human diseases from cancer to neurological has been well documented [72]. However, as we’ve seen some studies show that serum curcumin levels can be increased significantly with properly designed curcumin therapies. In addition, as serum curcumin/oid levels rise, in just hours the curcumin autooxidative degradation by-products can accompany the parent molecules in systemic circulation to contribute synergistic and/or additive pharmacology. This cannot be discounted.
Other challenges seem to compound the curcuminoid mystery including the lack of curcumin extract standardization. Curcumin extracts are notoriously comprised of varying proportions of the naturally inherent curcuminoid analogues. This in itself produces another layer of inconsistency when testing one curcumin standard against others. Lastly, the reports of the curcuminoid pharmacokinetics in the literature are conflicting and this is expected to be a function of the varying conditions influencing degradation of the variable curcuminoid proportions in multiple additive ways starting with formulation design and delivery form of the curcumin-therapy. This variability continues based on transition time and oxidative status in the lumen to interactions of different biochemicals used in the analysis of blood work as portrayed in Figure 2. Our very attempts to isolate, extract and assay these compounds produces degradation vulnerability that impairs accurate evaluation of curcumin/oid pharmacokinetics.
Everything from formulation, delivery form, Intestinal transition rate, diet, serum extraction method and serum storage and analysis can play a role in altering perceived bioavailability and serum stability. These represent multiple sources of variance and conflict from researcher to researcher.
In fact, we believe the very in vivo pharmacologically active biochemicals have been grossly missed in the past but have not been considered even in tissue distribution analysis – an endeavor that is so easily measured if one accepted the autodegradation by-products as a plausible source for, at least, part of the curcumin/oid pharmacology. These by-products are relatively stable in aqueous solution where the parent curcuminoids are not. We believe this to more than just plausible; it is cautiously expected to be highly likely.
The future requires a completely different outlook; first off by accepting polypharmacology or Network Pharmacology as a viable drug model by which the accepted Systems Biology is addressed with a pharmacological model that fits it like a glove. Secondly, the possibility that the degradation by-products are playing a significant role in one way or another in the expansive curcumin polypharmacology should be seriously investigated. The role that tetrahydrocurcumin might be playing must also be considered. Tissue distribution analysis must be employed with this objective in mind; and with clear consideration of the degradation potential inherent in the analytical process, itself. After this dust settles we’ll get to a starting line and determine what it is we are really studying and with this curcumin may get the credit it deserves even in mainstream medicine.
Conflict of Interest
One of the authors, Franco Cavaleri, is the owner of a corporation that funds and executes research on nutraceutical pharmacology and experimental medicine and has been involved in the research of curcuminoid pharmacology.
References
- Jurenka JS (2009) Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev 14: 141-153.
- Chattopadhyay I, Biswas K, Bandyopadhyay U, Banerjee RK (2004) Turmeric and curcumin: biological actions and medicinal applications. Current science 87: 44-53.
- Maheshwari RK, Singh AK, Gaddipati J, Srimal RC (2006) Multiple biological activities of curcumin: A short review. Life Sci 78: 2081-2087.
- Negi PS, Jayaprakasha GK, Jagan Mohan Rao L, Sakariah KK (1999) Antibacterial Activity of Turmeric Oil: A Byproduct from Curcumin Manufacture. J Agric Food Chem 47: 4297-4300.
- Gota VS, Maru GB, Soni TG, Gandhi TR, Kochar N, et al. (2010) Safety and Pharmacokinetics of a Solid Lipid Curcumin Particle Formulation in Osteosarcoma Patients and Healthy Volunteers. J Agric Food Chem 58: 2095-2099.
- Chandran B, Goel A (2012) Pilot Study to Assess the Efficacy and Safety of Curcumin in Patients with Active Rheumatoid Arthritis. Phytother Res 26: 1719-1725.
- Hsu C.H, A.L. Cheng (2007) Clinical studies with curcumin, in The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease. Springer 471- 480.
- Leclercq IA, Farrell GC, Sempoux C, Peña A dela, Horsmans Y et al. (2004) Curcumin inhibits NF-κB activation and reduces the severity of experimental steatohepatitis in mice. J Hepatol 41: 926-934.
- Chen Y-R, Tan TH (1998) Inhibition of the c-Jun N-terminal kinase (JNK) signaling pathway by curcumin. Oncogene 17: 173-178.
- Woo MS, Jung SH, Kim SY, Hyun JW, Ko KH, et al. (2005) Curcumin suppresses phorbol ester-induced matrix metalloproteinase-9 expression by inhibiting the PKC to MAPK signaling pathways in human astroglioma cells. Biochem Biophys Res Commun 335: 1017-1025.
- Sara M. Johnson, Pat Gulhati, Isela Arrieta, Xiaofu Wang, Tatsuo Uchida, et al. (2009)Curcumin Inhibits Proliferation of Colorectal Carcinoma by Modulating Akt/mTOR Signaling. Anticancer Res 29: 3185-3190.
- Yu S, Shen G, Khor TO, Kim JH, Kong ANT, et al. (2008) Curcumin inhibits Akt/mammalian target of rapamycin signaling through protein phosphatase-dependent mechanism. Mol Cancer Ther 7: 2609-2620.
- Binion DG, Otterson MF, Rafiee P (2008) Curcumin inhibits VEGF-mediated angiogenesis in human intestinal microvascular endothelial cells through COX-2 and MAPK inhibition. Gut 57:1509-1517.
- Clark EA, Leach KL, Trojanowski JQ, Lee VM (1991) Characterization and differential distribution of the three major human protein kinase C isozymes (PKC alpha, PKC beta, and PKC gamma) of the central nervous system in normal and Alzheimer's disease brains. Lab Invest 64: 35-44.
- O'Neill C, Cowburn RF, Bonkale WL, Ohm TG, Fastbom J, et al. (2001) Dysfunctional intracellular calcium homoeostasis: a central cause of neurodegeneration in Alzheimer's disease. Biochem Soc Symp 67: 177-194.
- Anderson K, Fitzgerald M, DuPont M, Wang T, Paz N, et al. (2006) Mice deficient in PKC theta demonstrate impaired in vivo T cell activation and protection from T cell-mediated inflammatory diseases. Autoimmunity 39: 469-478.
- Manicassamy S, Gupta S, Sun Z (2006) Selective function of PKC-theta in T cells. Cell Mol Immunol 3: 263-270.
- Brown MA, Jones WK (2004) NF-kappaB action in sepsis: the innate immune system and the heart. Front Biosci 9: 1201-1217.
- O’Connor AM, Bennett CL, Stacey D, Barry M, Col NF, et al. (2009) Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 8:3.
- Anand P, Kunnumakara AB, Sundaram C, Harikumar KB, Tharakan ST, et al. (2008) Cancer is a preventable disease that requires major lifestyle changes. Pharmaceutical research. Pharm Res 25: 2097-2116.
- Lin JK (2007) Molecular targets of curcumin. In: Aggarwal BB, Surh YJ, Shishodia S (eds) The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease. Springer, NY, USA. pp. 227- 243.
- El-Husseini A, Sabry A (2010) Fatal hyperhemolytic delayed transfusion reaction in sickle cell disease: A case report and literature review. Am J Emerg Med 28: 1062.e5-1062.e8.
- Farooqi A, Hou MF, Chen CC, Wang CL, Chang HW, et al. (2014) Androgen receptor and gene network: Micromechanics reassemble the signaling machinery of TMPRSS2-ERG positive prostate cancer cells. Cancer Cell Int 14: 34.
- Plummer SM, Holloway KA, Manson MM, Munks RJ, Kaptein A, et al. (1999) Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-κB activation via the NIK/IKK signalling complex. Oncogene 28: 18.
- Davis JM, Murphy EA, Carmichael MD, Zielinski MR, Groschwitz CM, et al.(2007) Curcumin effects on inflammation and performance recovery following eccentric exercise-induced muscle damage. Am J Physiol-Reg I 292: 2168-2173.
- Skrzypczak-Jankun E, McCabe NP, Selman SH, Jankun J (2000) Curcumin inhibits lipoxygenase by binding to its central cavity: theoretical and X-ray evidence. Int J Mol Med 6: 521-527.
- Huang MT, Lysz T, Ferraro T, Abidi TF, Laskin JD, et al. (1991) Inhibitory effects of curcumin on in vitro lipoxygenase and cyclooxygenase activities in mouse epidermis. Cancer Res 51: 813-819.
- Yang C, Zhang X, Fan H, Liu Y (2009) Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res 1282: 133-141.
- Bierhaus A, Zhang Y, Quehenberger P, Luther T, Haase M, et al. (1997) The dietary pigment curcumin reduces endothelial tissue factor gene expression by inhibiting binding of AP-1 to the DNA and activation of NF-kappa B. Thromb Haemost 77: 772-782.
- Xu YX, Pindolia KR, Janakiraman N, Chapman RA, Gautam SC, et al.(1997) Curcumin inhibits IL1 alpha and TNF-alpha induction of AP-1 and NF-kB DNA-binding activity in bone marrow stromal cells. Hematopathol Mol Hematol 11: 49-62.
- Bharti AC, Donato N, Aggarwal BB (2003) Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. The Journal of Immunology 171: 3863-3871.
- Rafiee P, Nelson VM, Manley S, Wellner M, Floer M, et al.(2009) Effect of curcumin on acidic pH-induced expression of IL-6 and IL-8 in human esophageal epithelial cells (HET-1A): role of PKC, MAPKs, and NF-κB. Am J Physiol Gastrointest Liver Physiol 296: 388-398.
- Cong Y, Wang L, Konrad A, Schoeb T, Elson CO, et al. (2009) Curcumin induces the tolerogenic dendritic cell that promotes differentiation of intestine-protective regulatory T cells. Eur J Immunol 39: 3134-3146.
- Larmonier CB, Uno JK, Lee K-M, Karrasch T, Laubitz D, et al.(2008) Limited effects of dietary curcumin on Th-1 driven colitis in IL-10 deficient mice suggest an IL-10-dependent mechanism of protection. Am J Physiol Gastrointest Liver Physiol 295: G1079-G1091.
- Shishodia S (2005) Curcumin: Getting Back to the Roots. Ann N Y Acad Sci 1056: 206-217.
- Yang KY, Lin LC, Tseng TY, Wang SC, Tsai TH, et al.(2007) Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC–MS/MS. J Chromatogr B 853: 183-189.
- Limtrakul P, Anuchapreeda S, Buddhasukh D (2004) Modulation of human multidrug-resistance MDR-1 gene by natural curcuminoids. BMC Cancer 17:4.
- Yue GGL, Cheng S-W, Yu H, Xu Z-S, Lee JKM, et al. (2012)The Role of Turmerones on Curcumin Transportation and P-Glycoprotein Activities in Intestinal Caco-2 Cells. J Med Food 15: 242-252.
- Lee HS (2006) Antiplatelet property of Curcuma longa L. rhizome-derived ar-turmerone. Bioresour Technol 97: 1372-1376.
- Tátraaljai D, Kirschweng B, Kovács J, Földes E, Pukánszky B, et al.(2013) Processing stabilisation of PE with a natural antioxidant, curcumin. Eur Polym J 49: 1196-1203.
- Metzler M, Pfeiffer E, Schulz SI, Dempe JS (2013) Curcumin uptake and metabolism. Biofactors 39: 14-20.
- Gordon ON, Schneider C (2012) Vanillin and ferulic acid: not the major degradation products of curcumin. Trends Mol Med 18: 361-363.
- Rasyid, Lelo (1999) The effect of curcumin and placebo on human gall-bladder function: an ultrasound study. Aliment Pharmacol Ther 13: 245-249.
- Akram M, Uddin S, Ahmed A, Usmanghani K, Hannan A,et al.(2010)Curcuma longa and curcumin: a review article. Rom J Biol–Plant Biol 55: 65-70.
- Bharti AC (2002) Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor-kappa B and Ikappa Balpha kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood 101: 1053-1062.
- Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, ET AL. (2001) Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res 21: 2895-2900.
- Agthong S, Kaewsema A, Charoensub T (2015) Curcumin Ameliorates Functional and Structural Abnormalities in Cisplatin-induced Neuropathy. Exp Neurobiol 24: 139.
- Lal B, Kapoor AK, Agrawal PK, Asthana OP, Srimal RC, et al. (2000) Role of curcumin in idiopathic inflammatory orbital pseudotumours. Phytother Res 14: 443-447.
- Kurd SK, Smith N, VanVoorhees A, Troxel AB, Badmaev V, et al. (2008) Oral curcumin in the treatment of moderate to severe psoriasis vulgaris: A prospective clinical trial. J Am Acad Dermatol 58: 625-631.
- Thangapazham RL, Sharma A, Maheshwari RK (2007) Beneficial role of curcumin in skin diseases. InThe Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease. Springer US 343-357.
- Baum L, Lam CW, Cheung SK, Kwok T, Lui V,et al. (2008) Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J Clin Psychopharmacol 28: 110-113.
- Garcia-Alloza M, Borrelli LA, Rozkalne A, Hyman BT, Bacskai BJ, et al. (2007) Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J Neurochem 102: 1095–1104.
- Mishra S, Palanivelu K (2008) The effect of curcumin (turmeric) on Alzheimer′s disease: An overview. Ann Indian Acad Neurol 11: 13.
- Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, et al. Curcumin Inhibits Formation of Amyloid β Oligomers and Fibrils, Binds Plaques, and Reduces Amyloid in vivo. J Biol Chem 280: 5892-5901.
- Ringman J, Frautschy S, Cole G, Masterman D, Cummings J et al. (2005) A Potential Role of the Curry Spice Curcumin in Alzheimers Disease. Curr Alzheimer Res 2: 131-136.
- Tripathi C, Anovadiya A, Barvaliya M, Shah R, Ghori V, et al. (2011) Adverse drug reaction profile of oseltamivir in Indian population: A prospective observational study. Indian J Pharmacol 43: 258.
- Hurley LL, Akinfiresoye L, Nwulia E, Kamiya A, Kulkarni AA, et al. (2013) Antidepressant-like effects of curcumin in WKY rat model of depression is associated with an increase in hippocampal BDNF. Behav Brain Res 239: 27-30.
- Cho JW, Lee KS, Kim CW (2007) Curcumin attenuates the expression of IL-1β, IL-6, and TNF-α as well as cyclin E in TNF-α-treated HaCaT cells; NF-κB and MAPKs as potential upstream targets. Int J Mol Med 19: 469-474.
- Maes M, Bosmans E, De Jongh R, Kenis G, Vandoolaeghe E, et al. (1997) Increased serum il-6 and il-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine 9: 853-858.
- Frommberger UH, Bauer J, Haselbauer P, Fräulin A, Riemann D, et al. (1997) Interleukin-6-(IL-6) plasma levels in depression and schizophrenia: comparison between the acute state and after remission. Eur Arch Psychiatry Clin Neurosci 247: 228-233.
- Wongcharoen W, Phrommintikul A (2009) The protective role of curcumin in cardiovascular diseases. Int J Cardiol 133: 145-151.
- Morimoto T, Sunagawa Y, Kawamura T, Takaya T, Wada H, et al. (2008) The dietary compound curcumin inhibits p300 histone acetyltransferase activity and prevents heart failure in rats. J Clin Invest 118: 868.
- Gharate MK. Rheumatoid (2007) Arthritis & Complementary and Alternative Medicine. Pharmainfo 5.
- Egan ME, Pearson M, Weiner SA, Rajendran V, Rubin D, et al. (2004) Curcumin, a major constituent of turmeric, corrects cystic fibrosis defects. Science 304: 600-602.
- Allam G. (2009) Immunomodulatory effects of curcumin treatment on murine Schistosomiasis mansoni. Immunobiology 214: 712-727.
- Moghaddam SJ, Barta P, Mirabolfathinejad SG, Ammar-Aouchiche Z, Garza NT, et al. (2009) Curcumin inhibits COPD-like airway inflammation and lung cancer progression in mice. Carcinogenesis 30: 1949-1956.
- Belkacemi A, Doggui S, Dao L, Ramassamy C (2011) Challenges associated with curcumin therapy in Alzheimer disease. Expert Rev Mol Med 13.
- Seo HJ, Wang SM, Han C, Lee SJ, Patkar AA, et al. (2015) Curcumin as a putative antidepressant. Expert Rev Neurother 15: 269-280.
- Mall M, Kunzelmann K (2005) Correction of the CF defect by curcumin: hypes and disappointments. Bioessays 27: 9-13.
- Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, et al. (2004) Phase I clinical trial of oral curcumin. Clin Cancer Res 10: 6847-6854.
- Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, et al. (2008) Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res 14: 4491-4499.
- Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB (2007) Bioavailability of curcumin: problems and promises. Mol Pharm 4: 807-818.
- Pan MH, Huang TM, Lin JK (1999) Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab Dispos 27: 486-494.
- Altunbas A, Lee SJ, Rajasekaran SA, Schneider JP, Pochan DJ et al. (2011) Encapsulation of curcumin in self-assembling peptide hydrogels as injectable drug delivery vehicles. Biomaterials 32: 5906-5914.
- Tsai YM, Jan WC, Chien CF, Lee WC, Lin LC, et al. (2011) Optimised nano-formulation on the bioavailability of hydrophobic polyphenol, curcumin, in freely-moving rats. Food Chem 127: 918-925.
- Holder GM, Plummer JL, Ryan AJ (1978) The metabolism and excretion of curcumin (1, 7-bis-(4-hydroxy-3-methoxyphenyl)-1, 6-heptadiene-3, 5-dione) in the rat. Xenobiotica 8: 761-768.
- Garcea G, Jones DJ, Singh R, Dennison AR, Farmer PB, et al. (2004) Detection of curcumin and its metabolites in hepatic tissue and portal blood of patients following oral administration. Br J Cancer 90(5): 1011-1015.
- Esmaili M, Ghaffari SM, Moosavi-Movahedi Z, Atri MS, Sharifizadeh A, et al. (2011) Beta casein-micelle as a nano vehicle for solubility enhancement of curcumin; food industry application. Food Sci Technol 44: 2166-2172.
- Yu H, Huang Q (2010) Enhanced in vitro anti-cancer activity of curcumin encapsulated in hydrophobically modified starch. Food Chem 119: 669-674.
- Kumar A, Ahuja A, Ali J, Baboota S (2010) Conundrum and therapeutic potential of curcumin in drug delivery. Crit Rev Ther Drug Carrier Syst 27.
- Belcaro G, Cesarone MR, Dugall M, Pellegrini L, Ledda A, et al. (2010) Efficacy and safety of Meriva (R), a curcumin-phosphatidylcholine complex, during extended administration in osteoarthritis patients. Altern Med Rev 15: 337-344.
- Khan J, Alexander A, Saraf S, Saraf S (2013) Recent advances and future prospects of phyto-phospholipid complexation technique for improving pharmacokinetic profile of plant actives. J Control Release 168: 50-60.
- Kim HJ, White PJ (2012) In vitro digestion rate and estimated glycemic index of oat flours from typical and high β-glucan oat lines. J Agric Food Chem 60: 5237-5242.
- Durback LF, Wedin GP, Seidler DE (1989) Management of lead foreign body ingestion. J Toxicol Clin Toxicol 27: 173-182.
- Benini L, Castellani G, Brighenti F, Heaton KW, et al. (1995) Gastric emptying of a solid meal is accelerated by the removal of dietary fibre naturally present in food. Gut 36: 825-830.
- Zhang G, Ao Z, Hamaker BR (2006) Slow digestion property of native cereal starches. Biomacromolecules 7: 3252-3258.
- Ireson CR, Jones DJ, Orr S, Coughtrie MW, Boocock DJ, et al. (2002) Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiol Biomarkers Prev 11: 105-111.
- Aggarwal BB, Sung B (2009) Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci 30: 85-94.
- Reddy AC, Sudharshan E, Rao AA, Lokesh BR (1999) Interaction of curcumin with human serum albumin—a spectroscopic study. Lipids 34: 1025-1029.
- Leung MH, Colangelo H, Kee TW (2008) Encapsulation of curcumin in cationic micelles suppresses alkaline hydrolysis. Langmuir 24: 5672-5675.
- Barik A, Priyadarsini KI, Mohan H (2003) Photophysical studies on binding of curcumin to bovine serum albumin. Photochem Photobiol 77: 597-603.
- Mitra SP (2007) Binding and stability of curcumin in presence of bovine serum albumin. Journal of Surface Science and Technology 23: 91.
- Zsila F, Bikádi Z, Simonyi M (2003) Unique, pH-dependent biphasic band shape of the visible circular dichroism of curcumin–serum albumin complex. Biochem Biophys Res Commun 301: 776-782.
- Hoehle SI, Pfeiffer E, Sólyom AM, Metzler M (2006) Metabolism of curcuminoids in tissue slices and subcellular fractions from rat liver. J Agric Food Chem 54: 756-764.
- Volak LP, Ghirmai S, Cashman JR (2008) Curcuminoids inhibit multiple human cytochromes P450, UDP-glucuronosyltransferase, and sulfotransferase enzymes, whereas piperine is a relatively selective CYP3A4 inhibitor. Drug Metab Dispos 36: 1594-1605.
- Ireson C, Orr S, Jones DJ, Verschoyle R, Lim CK, et al. (2001) Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res 61: 1058-1064.
- Cawley GF, Batie CJ, Backes WL (1995) Substrate-dependent competition of different P 450 isoenzymes for limiting NADPH-cytochrome P 450 reductase. Biochemistry 34: 1244-1247.
- Das A, Sligar SG (2009) Modulation of the cytochrome P450 reductase redox potential by the phospholipid bilayer. Biochemistry 48: 12104-12112.
- Shank-Retzlaff ML, Raner GM, Coon MJ, Sligar SG (1998) Membrane topology of cytochrome P450 2B4 in Langmuir–Blodgett monolayers. Arch Biochem Biophys 359: 82-88.
- Yallapu MM, Ebeling MC, Chauhan N, Jaggi M, Chauhan SC et al. (2011) Interaction of curcumin nanoformulations with human plasma proteins and erythrocytes. Int J Nanomedicine 6: 2779.
- Bisht S, Maitra A (2009) Systemic delivery of curcumin: 21st century solutions for an ancient conundrum. Curr Drug Discov Technol 6: 192-199.
- Wang YJ, Pan MH, Cheng AL, Lin LI, Ho YS, et al. ( 1997) Stability of curcumin in buffer solutions and characterization of its degradation products. J Pharm Biomed Anal 15: 1867-1876.
- Babu RO, Moorkoth D, Azeez S, Eapen SJ (2012) Virtual screening and in vitro assay of potential drug like inhibitors from spices against Glutathione-S-Transferase of Meloidogyne incognita. Bioinformation 8: 319.
- Ota A, AbramoviÃÆââ¬Å¾ÃâàH, Abram V, Ulrih NP (2011) Interactions of p-coumaric, caffeic and ferulic acids and their styrenes with model lipid membranes. Food Chem 125: 1256-1261.
- Shen L, Ji HF (2012) The pharmacology of curcumin: is it the degradation products?. Trends Mol Med 18: 138-144.
- Kurien BT, Scofield RH (2007) Curcumin/turmeric solubilized in sodium hydroxide inhibits HNE protein modification—an in vitro study. J Ethnopharmacol 110: 368-373.
- Shen L, Ji HF (2009) Contribution of degradation products to the anticancer activity of curcumin. Clin Cancer Res 15: 7108.
- Liang G, Shao L, Wang Y, Zhao C, Chu Y, et al. (2009) Exploration and synthesis of curcumin analogues with improved structural stability both in vitro and in vivo as cytotoxic agents. Bioorg Med Chem Lett 17: 2623-2631.
- Aggarwal BB, Harikumar KB (2009) Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol 41: 40-59.
- Epstein J, Sanderson IR, MacDonald TT (2010) Curcumin as a therapeutic agent: the evidence from in vitro, animal and human studies. Br J Nutr 103: 1545-1557.
- Henrotin Y, Clutterbuck AL, Allaway D, Lodwig EM, Harris P, et al. (2010) Biological actions of curcumin on articular chondrocytes. Osteoarthritis Cartilage 18: 141-149.
- ABE Y, Hashimoto SH, HORIE T (1999) Curcumin inhibition of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophages. Pharmacol Res 39: 41-47.
- Sugiyama Y, Kawakishi S, Osawa T (1996) Involvement of the β-diketone moiety in the antioxidative mechanism of tetrahydrocurcumin. Biochem Pharmacol 52: 519-525.
- Pari L, Murugan P (2007) Antihyperlipidemic effect of curcumin and tetrahydrocurcumin in experimental type 2 diabetic rats. Ren Fail 29: 881-889.
- Kumar M, Givvimani S, Puspakumar SB, Mishra PK, Kundu S, et al. (2009) Cerebroprotective role of Tetrahydro Curcumin in hyperhomocysteinemic ischemic mice by regulating NF-kappa B. Faseb J 23: 614-617.
- Mukhopadhyay A, Basu N, Ghatak N, Gujral PK (1982) Anti-inflammatory and irritant activities of curcumin analogues in rats. Inflamm Res 12: 508-515.
- Sandur SK, Pandey MK, Sung B, Ahn KS, Murakami A, et al. (2007) Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis 28: 1765-1773.
- Murakami Y, Ishii H, Takada N, Tanaka S, MACHINO M, et al. (2008) Comparative anti-inflammatory activities of curcumin and tetrahydrocurcumin based on the phenolic OH bond dissociation enthalpy, ionization potential and quantum chemical descriptor. Anticancer Res 28: 699-707.
- Yanagisawa D, Shirai N, Amatsubo T, Taguchi H, Hirao K, et al. (2010) Relationship between the tautomeric structures of curcumin derivatives and their Aβ-binding activities in the context of therapies for Alzheimer's disease. Biomaterials 31: 4179-4185.
- Payton F, Sandusky P, Alworth WL (2007) NMR study of the solution structure of curcumin. J Nat Prod 70: 143-146.
- Jovanovic SV, Steenken S, Boone CW, Simic MG (1999) H-atom transfer is a preferred antioxidant mechanism of curcumin. J Am Chem Soc 121: 9677-9681.
- Lin JK, Pan MH, Lin-Shiau SY (2000) Recent studies on the biofunctions and biotransformations of curcumin. Biofactors 13: 153-158.
- Martelli L, Ragazzi E, Di Mario F, Martelli M, Castagliuolo I, et al. (2007) A potential role for the vanilloid receptor TRPV1 in the therapeutic effect of curcumin in dinitrobenzene sulphonic acidÃÆâÃâââ¬ÃâÃÂinduced colitis in mice. Neurogastroenterol Motil 19: 668-674.
- Yamamoto M, Yoshizaki K, Kishimoto T, Ito H (2000) IL-6 is required for the development of Th1 cell-mediated murine colitis. J Immunol 164: 4878-4882.
- Ferretti M, Casini-Raggi V, Pizarro TT, Eisenberg SP, Nast CC, et al. (1994) Neutralization of endogenous IL-1 receptor antagonist exacerbates and prolongs inflammation in rabbit immune colitis. J Clin Invest 94: 449.
- Neurath MF, Pettersson S, Zum Büschenfelde KH, Strober W (1996) Local administration of antisense phosphorothiate olignucleotides to the p65 subunit of NF–κB abrogates established experimental colitis in mice. Nat Med 2: 998-1004.
- Kim MC, Kim SJ, Kim DS, Jeon YD, Park SJ, et al. (2011) Vanillic acid inhibits inflammatory mediators by suppressing NF-κB in lipopolysaccharide-stimulated mouse peritoneal macrophages. Immunopharmacol Immunotoxicol 33: 525-532.
- Goel A, Boland CR, Chauhan DP (2001) Specific inhibition of cyclooxygenase-2 (COX-2) expression by dietary curcumin in HT-29 human colon cancer cells. Cancer Lett 172: 111-118.
- Cho JW, Park K, Kweon GR, Jang BC, Baek WK, et al. (2005) Curcumin inhibits the expression of COX-2 in UVB-irradiated human keratinocytes (HaCaT) by inhibiting activation of AP-1: p38 MAP kinase and JNK as potential upstream targets. Exp Mol Med 37: 186-192.
- Kanski J, Aksenova M, Stoyanova A, Butterfield DA (2002) Ferulic acid antioxidant protection against hydroxyl and peroxyl radical oxidation in synaptosomal and neuronal cell culture systems in vitro: structure-activity studies. J Nutr Biochem 13: 273-281.
- Scapagnini G, Colombrita C, Amadio M, D'Agata V, Arcelli E, et al. (2006) Curcumin activates defensive genes and protects neurons against oxidative stress. Antioxid Redox Signal 8: 395-403.
- Suzuki A, Kagawa D, Fujii A, Ochiai R, Tokimitsu I, et al. (2002) Short-and long-term effects of ferulic acid on blood pressure in spontaneously hypertensive rats. Am J Hypertens 15: 351-357.
- HlavaÃÆââ¬Å¾ÃâÃÂková L, Janegová A, UliÃÆââ¬Å¾ÃâÃÂná O, Janega P, ÃÆââ¬Å¾Ãâà âerná A, et al. (2011) Spice up the hypertension diet-curcumin and piperine prevent remodeling of aorta in experimental L-NAME induced hypertension. Nutr Metab 8: 72.
- Yang F, Zhou BR, Zhang P, Zhao YF, Chen Jet al. (2007) Binding of ferulic acid to cytochrome c enhances stability of the protein at physiological pH and inhibits cytochrome c-induced apoptosis. Chem Biol Interact 170: 231-243.
- Kampa M, Alexaki VI, Notas G, Nifli AP, Nistikaki A, et al. (2003) Antiproliferative and apoptotic effects of selective phenolic acids on T47D human breast cancer cells: potential mechanisms of action. Breast Cancer Res 6: R63-R74.
- Huang TY, Hsu CW, Chang WC, Wang MY, Wu JF et al. (2012) Demethoxycurcumin retards cell growth and induces apoptosis in human brain malignant glioma GBM 8401 cells. Evid-Based Compl Alt.
- Reinke AA, Gestwicki JE (2007) Structure–activity Relationships of Amyloid BetaÃÆâÃâââ¬ÃâÃÂaggregation Inhibitors Based on Curcumin: Influence of Linker Length and Flexibility. Chem Biol Drug Des 70: 206-125.
- Park SY, Kim HS, Cho EK, Kwon BY, Phark S, et al. (2008) Curcumin protected PC12 cells against beta-amyloid-induced toxicity through the inhibition of oxidative damage and tau hyperphosphorylation. Food Chem Toxicol 46: 2881-2887.
- Hamaguchi T, Ono K, Murase A, Yamada M (2009) Phenolic compounds prevent Alzheimer’s pathology through different effects on the amyloid-β aggregation pathway. The Am J Pathol 175: 2557-2565.
- Jobin C, Bradham CA, Russo MP, Juma B, Narula AS, et al. (1999) Curcumin blocks cytokine-mediated NF-κB activation and proinflammatory gene expression by inhibiting inhibitory factor I-κB kinase activity. J Immunol 163: 3474-3483.
- Menon VP, Sudheer AR (2007) Antioxidant and anti-inflammatory properties of curcumin. InThe molecular targets and therapeutic uses of curcumin in health and disease. Springer 15: 105-125.
- Sultana R, Ravagna A, MohmmadÃÆâÃâââ¬ÃâÃÂAbdul H, Calabrese V, Butterfield DA et al. (2005) Ferulic acid ethyl ester protects neurons against amyloid βÃÆâÃâââ¬ÃâÃÂpeptide (1-42)ÃÆâÃâââ¬ÃâÃÂinduced oxidative stress and neurotoxicity: relationship to antioxidant activity. J Neurochem 92: 749-758.
- Ono K, Hirohata M, Yamada M (2005) Ferulic acid destabilizes preformed β-amyloid fibrils in vitro. Biochem Biophys Res Commun 336: 444-449.
- Silverman RB, Holladay MW (2014) The organic chemistry of drug design and drug action. Academic press.
- Pajouhesh H, Lenz GR (2005) Medicinal chemical properties of successful central nervous system drugs. NeuroRx 2: 541-553.
- Masoudi-Nejad A, Mousavian Z, Bozorgmehr JH (2013) Drug-target and disease networks: polypharmacology in the post-genomic era. In Silico Pharmacol 1: 17.
- Ma B (2014) Editorial (Thematic Issue: Protein-protein interaction: from interface to interaction network). Curr Pharm Des 20: 1171-1172.
- Flower DR (2013) The Immune System as Drug Target. Immunol Immunogenet Insights 5: 1.
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, et al. (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350: 2335-2342.
- Onoue S, Takahashi H, Kawabata Y, Seto Y, Hatanaka J, et al. (2010) Formulation design and photochemical studies on nanocrystal solid dispersion of curcumin with improved oral bioavailability. J Pharm Sci 99: 1871-1881.
- Canamares MV, Garcia-Ramos JV, Sanchez-Cortes S (2006) Degradation of curcumin dye in aqueous solution and on Ag nanoparticles studied by ultraviolet–visible absorption and surface-enhanced Raman spectroscopy. Appl Spectrosc 60: 1386-1391.
- Bosch E, Bou P, Allemann H, Rosés M (1996) Retention of Ionizable Compounds on HPLC. pH Scale in Methanol− Water and the p K and pH Values of Buffers. Anal Chem 68: 3651-3657.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences